SCF-SKP2 E3 ubiquitin ligase links mTORC1/ER stress/ISR with YAP activation in murine renal cystogenesis
- PMID: 36326820
- PMCID: PMC9754004
- DOI: 10.1172/JCI153943
SCF-SKP2 E3 ubiquitin ligase links mTORC1/ER stress/ISR with YAP activation in murine renal cystogenesis
Abstract
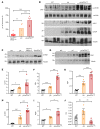
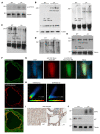
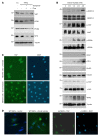
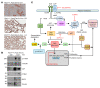
The Hippo pathway nuclear effector Yes-associated protein (YAP) potentiates the progression of polycystic kidney disease (PKD) arising from ciliopathies. The mechanisms underlying the increase in YAP expression and transcriptional activity in PKD remain obscure. We observed that in kidneys from mice with juvenile cystic kidney (jck) ciliopathy, the aberrant hyperactivity of mechanistic target of rapamycin complex 1 (mTORC1), driven by ERK1/2 and PI3K/AKT cascades, induced ER proteotoxic stress. To reduce this stress by reprogramming translation, the protein kinase R-like ER kinase-eukaryotic initiation factor 2α (PERK/eIF2α) arm of the integrated stress response (ISR) was activated. PERK-mediated phosphorylation of eIF2α drove the selective translation of activating transcription factor 4 (ATF4), potentiating YAP expression. In parallel, YAP underwent K63-linked polyubiquitination by SCF S-phase kinase-associated protein 2 (SKP2) E3 ubiquitin ligase, a Hippo-independent, nonproteolytic ubiquitination that enhances YAP nuclear trafficking and transcriptional activity in cancer cells. Defective ISR cellular adaptation to ER stress in eIF2α phosphorylation-deficient jck mice further augmented YAP-mediated transcriptional activity and renal cyst growth. Conversely, pharmacological tuning down of ER stress/ISR activity and SKP2 expression in jck mice by administration of tauroursodeoxycholic acid (TUDCA) or tolvaptan impeded these processes. Restoring ER homeostasis and/or interfering with the SKP2-YAP interaction represent potential therapeutic avenues for stemming the progression of renal cystogenesis.
Keywords: Chronic kidney disease; Nephrology.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

