One-year follow-up of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients
- PMID: 36326824
- PMCID: PMC9753994
- DOI: 10.1172/JCI163657
One-year follow-up of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients
Abstract
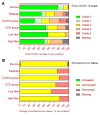
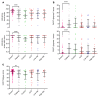
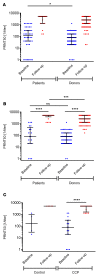
BACKGROUNDResults of many randomized trials on COVID-19 convalescent plasma (CCP) have been reported, but information on long-term outcome after CCP treatment is limited. The objectives of this extended observation of the randomized CAPSID trial are to assess long-term outcome and disease burden in patients initially treated with or without CCP.METHODSOf 105 randomized patients, 50 participated in the extended observation. Quality of life (QoL) was assessed by questionnaires and a structured interview. CCP donors (n = 113) with asymptomatic to moderate COVID-19 were included as a reference group.RESULTSThe median follow-up of patients was 396 days, and the estimated 1-year survival was 78.7% in the CCP group and 60.2% in the control (P = 0.08). The subgroup treated with a higher cumulative amount of neutralizing antibodies showed a better 1-year survival compared with the control group (91.5% versus 60.2%, P = 0.01). Medical events and QoL assessments showed a consistent trend for better results in the CCP group without reaching statistical significance. There was no difference in the increase in neutralizing antibodies after vaccination between the CCP and control groups.CONCLUSIONThe trial demonstrated a trend toward better outcome in the CCP group without reaching statistical significance. A predefined subgroup analysis showed a significantly better outcome (long-term survival, time to discharge from ICU, and time to hospital discharge) among those who received a higher amount of neutralizing antibodies compared with the control group. A substantial long-term disease burden remains after severe COVID-19.Trial registrationEudraCT 2020-001310-38 and ClinicalTrials.gov NCT04433910.FundingBundesministerium für Gesundheit (German Federal Ministry of Health).
Keywords: COVID-19; Immunotherapy; Therapeutics.
Conflict of interest statement
Figures