The COVID HOME study research protocol: Prospective cohort study of non-hospitalised COVID-19 patients
- PMID: 36327223
- PMCID: PMC9632784
- DOI: 10.1371/journal.pone.0273599
The COVID HOME study research protocol: Prospective cohort study of non-hospitalised COVID-19 patients
Abstract
Background: Guidelines on COVID-19 management are developed as we learn from this pandemic. However, most research has been done on hospitalised patients and the impact of the disease on non-hospitalised and their role in transmission are not yet well understood. The COVID HOME study conducts research among COVID-19 patients and their family members who were not hospitalised during acute disease, to guide patient care and inform public health guidelines for infection prevention and control in the community and household.
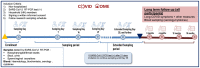
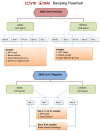
Methods: An ongoing prospective longitudinal observational study of COVID-19 outpatients was established in March 2020 at the beginning of the COVID-19 pandemic in the Netherlands. Laboratory confirmed SARS-CoV-2 infected individuals of all ages that did not merit hospitalisation, and their household (HH) members, were enrolled after written informed consent. Enrolled participants were visited at home within 48 hours after initial diagnosis, and then weekly on days 7, 14 and 21 to obtain clinical data, a blood sample for biochemical parameters/cytokines and serological determination; and a nasopharyngeal/throat swab plus urine, stool and sperm or vaginal secretion (if consenting) to test for SARS-CoV-2 by RT-PCR (viral shedding) and for viral culturing. Weekly nasopharyngeal/throat swabs and stool samples, plus a blood sample on days 0 and 21 were also taken from HH members to determine whether and when they became infected. All participants were invited to continue follow-up at 3-, 6-, 12- and 18-months post-infection to assess long-term sequelae and immunological status.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Absence of SARS-CoV-2 infection in the semen of men recovering from COVID-19 infection: An exploratory study and review of literature.Andrologia. 2021 Sep;53(8):e14136. doi: 10.1111/and.14136. Epub 2021 Jun 11. Andrologia. 2021. PMID: 34115901 Free PMC article.
-
Incidence and mortality due to thromboembolic events during the COVID-19 pandemic: Multi-sourced population-based health records cohort study.Thromb Res. 2021 Jun;202:17-23. doi: 10.1016/j.thromres.2021.03.006. Epub 2021 Mar 8. Thromb Res. 2021. PMID: 33711754 Free PMC article.
Cited by
-
Temporal Dynamics and (Para)Clinical Factors Associated With (Long) Viral RNA Shedding in COVID-19 Nonhospitalized Individuals - The COVID-HOME Study.J Med Virol. 2024 Dec;96(12):e70125. doi: 10.1002/jmv.70125. J Med Virol. 2024. PMID: 39690930 Free PMC article.
-
The gut microbiota as an early predictor of COVID-19 severity.mSphere. 2024 Oct 29;9(10):e0018124. doi: 10.1128/msphere.00181-24. Epub 2024 Sep 19. mSphere. 2024. PMID: 39297639 Free PMC article.
References
-
- RIVM (National Institute for Public Health and the Environment). Patient with novel coronavirus COVID-19 in the Netherlands. 2020 [cited 27-02-2020]. https://www.rivm.nl/node/152811.
-
- Coronavirus Dashboard—Deaths [Internet]. Rijksoverheid (Government of the Netherlands). 2022. https://coronadashboard.government.nl/landelijk/sterfte.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

