Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study
- PMID: 36327426
- PMCID: PMC9937097
- DOI: 10.1200/JCO.22.00975
Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study
Abstract
Purpose: The open-label, phase III POSEIDON study evaluated tremelimumab plus durvalumab and chemotherapy (T + D + CT) and durvalumab plus chemotherapy (D + CT) versus chemotherapy alone (CT) in first-line metastatic non-small-cell lung cancer (mNSCLC).
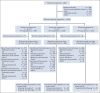
Methods: Patients (n = 1,013) with EGFR/ALK wild-type mNSCLC were randomly assigned (1:1:1) to tremelimumab 75 mg plus durvalumab 1,500 mg and platinum-based chemotherapy for up to four 21-day cycles, followed by durvalumab once every 4 weeks until progression and one additional tremelimumab dose; durvalumab plus chemotherapy for up to four 21-day cycles, followed by durvalumab once every 4 weeks until progression; or chemotherapy for up to six 21-day cycles (with or without maintenance pemetrexed; all arms). Primary end points were progression-free survival (PFS) and overall survival (OS) for D + CT versus CT. Key alpha-controlled secondary end points were PFS and OS for T + D + CT versus CT.
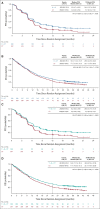
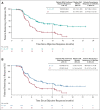
Results: PFS was significantly improved with D + CT versus CT (hazard ratio [HR], 0.74; 95% CI, 0.62 to 0.89; P = .0009; median, 5.5 v 4.8 months); a trend for improved OS did not reach statistical significance (HR, 0.86; 95% CI, 0.72 to 1.02; P = .0758; median, 13.3 v 11.7 months; 24-month OS, 29.6% v 22.1%). PFS (HR, 0.72; 95% CI, 0.60 to 0.86; P = .0003; median, 6.2 v 4.8 months) and OS (HR, 0.77; 95% CI, 0.65 to 0.92; P = .0030; median, 14.0 v 11.7 months; 24-month OS, 32.9% v 22.1%) were significantly improved with T + D + CT versus CT. Treatment-related adverse events were maximum grade 3/4 in 51.8%, 44.6%, and 44.4% of patients receiving T + D + CT, D + CT, and CT, respectively; 15.5%, 14.1%, and 9.9%, respectively, discontinued treatment because of treatment-related adverse events.
Conclusion: D + CT significantly improved PFS versus CT. A limited course of tremelimumab added to durvalumab and chemotherapy significantly improved OS and PFS versus CT, without meaningful additional tolerability burden, representing a potential new option in first-line mNSCLC.
Trial registration: ClinicalTrials.gov NCT03164616.
Figures


Comment in
-
First-line management of advanced non-small-cell lung cancer: can we do better?Transl Lung Cancer Res. 2023 Jul 31;12(7):1643-1648. doi: 10.21037/tlcr-23-200. Epub 2023 Jun 1. Transl Lung Cancer Res. 2023. PMID: 37577310 Free PMC article. No abstract available.
References
-
- Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 - PubMed
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. : Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078-2092, 2018 - PubMed
-
- Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 - PubMed
-
- Socinski MA, Jotte RM, Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 - PubMed
-
- West H, McCleod M, Hussein M, et al. : Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924-937, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

