Clozapine prescription trends in Brazil in the last decade
- PMID: 36327429
- PMCID: PMC9851753
- DOI: 10.47626/1516-4446-2022-2572
Clozapine prescription trends in Brazil in the last decade
Abstract
Objective: Clozapine is a second-generation antipsychotic indicated for treatment-resistant schizophrenia. Studies in several countries have shown a low rate of clozapine use despite the fact that approximately 30% of schizophrenia cases are treatment-resistant. In Brazil, few studies have addressed the frequency and variety of antipsychotic use in individuals diagnosed with schizophrenia (ICD F20). The objective of this study was to measure the rates of clozapine use in this population in the last decade using Brazilian Ministry of Health data.
Methods: Prescriptions made between 2010 and 2020 in all 26 states and the Federal District registered at the Outpatient Information System Database from the Brazilian Health System (SIASUS) were evaluated.
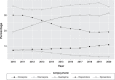
Results: A total of 25,143,524 prescriptions were recorded in this period, with clozapine representing 8.86% of all antipsychotics. The most frequently prescribed antipsychotic for patients with schizophrenia was olanzapine (35.8%), followed by quetiapine (27.5%). From 2010 to 2020, the rate of clozapine prescriptions in Brazil increased from 7.2% to 10.9%.
Conclusions: Despite a slight increase in prescriptions in the last decade, clozapine is still underutilized in Brazil.
Keywords: Clozapine; antipsychotics; schizophrenia; treatment-resistant.
Conflict of interest statement
RM has been a consultant/advisor and/or has received honoraria from Daiichi-Sankyo and Janssen. HE has received honoraria for participation as a member of advisory boards, speaker, or travel support from the following pharmaceutical companies: Aché, Cristalia, Daiichi-Sankyo, Janssen, Mantecorp-Hypera, Sandoz, and Teva. DFL has been a consultant/advisor and/or has received honoraria from EMS, Daiichi-Sankyo, Servier, and Greencare. RB has received personal fees from Torrent, Lundbeck, and Ache, and personal fees and non-financial support from Janssen, outside the submitted work. CN has been a consultant/advisor and/or has received honoraria from Ache´, Daiichi-Sankyo, Teva, and Janssen. AG has been a consultant/advisor and/or has received honoraria from Ache´, Daiichi-Sankyo, Torrent, Cristalia, and Janssen. The other authors report no conflicts of interest.
Figures
References
-
- Elkis H, Buckley PF. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2016;39:239–65. - PubMed
-
- Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. 2019;76:1052–62. - PMC - PubMed
-
- Brasil, Ministério da Saúde Portaria SAS/MS no 364, de 9 de abril de 2013. bvsms.saude.gov.br/bvs/saudelegis/sas/2013/prt0364_09_04_2013.html
MeSH terms
Substances
LinkOut - more resources
Full Text Sources