A moving target for drug discovery: Structure activity relationship and many genome (de)stabilizing functions of the RAD52 protein
- PMID: 36327799
- PMCID: PMC9888176
- DOI: 10.1016/j.dnarep.2022.103421
A moving target for drug discovery: Structure activity relationship and many genome (de)stabilizing functions of the RAD52 protein
Abstract
BRCA-ness phenotype, a signature of many breast and ovarian cancers, manifests as deficiency in homologous recombination, and as defects in protection and repair of damaged DNA replication forks. A dependence of such cancers on DNA repair factors less important for survival of BRCA-proficient cells, offers opportunities for development of novel chemotherapeutic interventions. The first drugs targeting BRCA-deficient cancers, poly-ADP-ribose polymerase (PARP) inhibitors have been approved for the treatment of advanced, chemotherapy resistant cancers in patients with BRCA1/2 germline mutations. Nine additional proteins that can be targeted to selectively kill BRCA-deficient cancer cells have been identified. Among them, a DNA repair protein RAD52 is an especially attractive target due to general tolerance of the RAD52 loss of function, and protective role of an inactivating mutation. Yet, the effective pharmacological inhibitors of RAD52 have not been forthcoming. In this review, we discuss advances in the state of our knowledge of the RAD52 structure, activities and cellular functions, with a specific focus on the features that make RAD52 an attractive, but difficult drug target.
Keywords: BRCA1; BRCA2; DNA double strand break repair; Homologous recombination; Pharmacological inhibition of protein-DNA interactions; RAD52; Synthetic lethality in cancer.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures
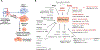


References
-
- Byrum AK, Vindigni A, Mosammaparast N, Defining and Modulating ‘BRCAness’, Trends in Cell Biology, 29 (2019) 740–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

