The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity
- PMID: 36327900
- PMCID: PMC9986959
- DOI: 10.1016/j.neuron.2022.10.017
The sympathetic nervous system in the 21st century: Neuroimmune interactions in metabolic homeostasis and obesity
Abstract
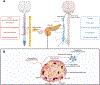
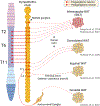
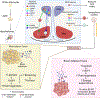
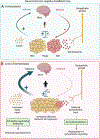
The sympathetic nervous system maintains metabolic homeostasis by orchestrating the activity of organs such as the pancreas, liver, and white and brown adipose tissues. From the first renderings by Thomas Willis to contemporary techniques for visualization, tracing, and functional probing of axonal arborizations within organs, our understanding of the sympathetic nervous system has started to grow beyond classical models. In the present review, we outline the evolution of these findings and provide updated neuroanatomical maps of sympathetic innervation. We offer an autonomic framework for the neuroendocrine loop of leptin action, and we discuss the role of immune cells in regulating sympathetic terminals and metabolism. We highlight potential anti-obesity therapeutic approaches that emerge from the modern appreciation of SNS as a neural network vis a vis the historical fear of sympathomimetic pharmacology, while shifting focus from post- to pre-synaptic targeting. Finally, we critically appraise the field and where it needs to go.
Keywords: adipose tissue; leptin; liver; metabolism; neuroimmunology; pancreas; sympathetic neurons.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- ADORI C, DARAIO T, KUIPER R, BARDE S, HORVATHOVA L, YOSHITAKE T, IHNATKO R, VALLADOLID-ACEBES I, VERCRUYSSE P, WELLENDORF AM, GRAMIGNOLI R, BOZOKY B, KEHR J, THEODORSSON E, CANCELAS JA, MRAVEC B, JORNS C, ELLIS E, MULDER J, UHLEN M, BARK C. & HOKFELT T. 2021. Disorganization and degeneration of liver sympathetic innervations in nonalcoholic fatty liver disease revealed by 3D imaging. Sci Adv, 7. - PMC - PubMed
-
- AHREN B, ERICSON LE, LUNDQUIST I, LOREN I. & SUNDLER F. 1981. Adrenergic innervation of pancreatic islets and modulation of insulin secretion by the sympatho-adrenal system. Cell Tissue Res, 216, 15–30. - PubMed
-
- AHREN B. & TABORSKY GJ JR. 1986. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology, 118, 1551–7. - PubMed
-
- AHREN B, VEITH RC & TABORSKY GJ JR. 1987. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1). Effects on basal release of insulin and glucagon. Endocrinology, 121, 323–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

