Ancestral library identifies conserved reprogrammable liver motif on AAV capsid
- PMID: 36327973
- PMCID: PMC9729830
- DOI: 10.1016/j.xcrm.2022.100803
Ancestral library identifies conserved reprogrammable liver motif on AAV capsid
Abstract
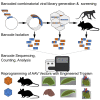
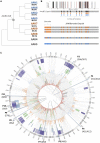
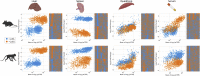
Gene therapy is emerging as a modality in 21st-century medicine. Adeno-associated viral (AAV) gene transfer is a leading technology to achieve efficient and durable expression of a therapeutic transgene. However, the structural complexity of the capsid has constrained efforts to engineer the particle toward improved clinical safety and efficacy. Here, we generate a curated library of barcoded AAVs with mutations across a variety of functionally relevant motifs. We then screen this library in vitro and in vivo in mice and nonhuman primates, enabling a broad, multiparametric assessment of every vector within the library. Among the results, we note a single residue that modulates liver transduction across all interrogated models while preserving transduction in heart and skeletal muscles. Moreover, we find that this mutation can be grafted into AAV9 and leads to profound liver detargeting while retaining muscle transduction-a finding potentially relevant to preventing hepatoxicities seen in clinical studies.
Keywords: AAV9; ASR; Anc80; adeno-associated virus; ancestral sequence reconstruction; capsid; gene therapy; hepatotoxicity; hepatotropism; library; liver toggle.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.H.V. holds equity in Affinia Therapeutics, Akouos, and ciendias bio. He serves on the Board of Directors of Affinia Therapeutics, Addgene, ciendias bio, and Odylia. L.H.V. is an employee of ciendias bio. L.H.V. and E.Z. are inventors of AncAAV technology licensed to Affinia, Akouos, and/or other biopharmaceutical companies from which they may receive royalties. L.H.V. is compensated for his scientific advisory position with Affinia and Akouos. L.H.V. is a SAB member to Akouos, consultant to Affinia and Novartis, and receives research support from Novartis. L.H.V.’s interests were reviewed and are managed by Mass Eye and Ear and Mass General Brigham in accordance with their conflict-of-interest policies. L.H.V., E.Z., C.U., P.F.S., C.T., and H.T.T. are inventors on patent applications relating to AAVSeq, CombiAAV, or the liver toggle technology contained in this publication. A.J.W. and L.H.V. are inventors on patents related to use of AAVs for therapeutic gene delivery in the muscle and other tissues.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

