GLPG1205 for idiopathic pulmonary fibrosis: a phase 2 randomised placebo-controlled trial
- PMID: 36328358
- PMCID: PMC9978158
- DOI: 10.1183/13993003.01794-2022
GLPG1205 for idiopathic pulmonary fibrosis: a phase 2 randomised placebo-controlled trial
Abstract
Background: GLPG1205 is a selective functional antagonist of G-protein-coupled receptor 84, which plays an important role in fibrotic processes. This study assessed the efficacy, safety and tolerability of GLPG1205 for treatment of idiopathic pulmonary fibrosis (IPF).
Methods: PINTA (ClinicalTrials.gov: NCT03725852) was a phase 2, randomised, double-blind, placebo-controlled, proof-of-concept trial. Patients with IPF were randomised 2:1 to once-daily oral GLPG1205 100 mg or placebo for 26 weeks and stratified to receive GLPG1205 alone or with local standard of care (nintedanib or pirfenidone). The primary end-point was change from baseline in forced vital capacity (FVC); other end-points were safety and tolerability, and lung volumes measured by imaging (high-resolution computed tomography). The study was not powered for statistical significance.
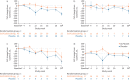
Results: In total, 68 patients received study medication. Least squares mean change from baseline in FVC at week 26 was -33.68 (95% CI -112.0-44.68) mL with GLPG1205 and -76.00 (95% CI -170.7-18.71) mL with placebo (least squares mean difference 42.33 (95% CI -81.84-166.5) mL; p=0.50). Lung volumes by imaging declined -58.30 versus -262.72 mL (whole lung) and -33.68 versus -135.48 mL (lower lobes) with GLPG1205 versus placebo, respectively. Treatment with GLPG1205 versus placebo resulted in higher proportions of serious and severe treatment-emergent adverse events and treatment-emergent discontinuations, most apparent with nintedanib.
Conclusions: Treatment with GLPG1205 did not result in a significant difference in FVC decline versus placebo. GLPG1205 demonstrated a poorer safety and tolerability profile than placebo.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: I.R. Strambu is an investigator in the PINTA study and received an investigator's fee and support for travel costs to a PINTA investigator meeting from Galapagos; she has also received investigator's fees from Novartis and GlaxoSmithKline; has been paid as a speaker by AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Roche and Teva; and has been an advisory board member for Boehringer Ingelheim. C.A. Seemayer, T.A.K. Van der Aa, A.A. de Haas-Amatsaleh and E. Santermans are employees of Galapagos and have received warrants from Galapagos. L.M-C.A. Fagard, E.N. Sondag, E.G. Helmer and P.A. Ford were employees of Galapagos at the time of the study and have received warrants from Galapagos. V. Modgill is an employee of Galapagos. T.M. Maher has, via his institution, received industry-academic funding from AstraZeneca and GlaxoSmithKline R&D; and has received consultancy and/or speakers’ fees from AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline R&D, IQVIA, Pliant, Respivant, Roche and Theravance. U. Costabel has received personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Fibrogen, Galapagos, Novartis, Pliant Therapeutics and Roche, and has served on data safety monitoring boards for Boehringer Ingelheim, Galapagos (including for the PINTA study), Roche and Sanofi. V. Cottin reports personal fees and non-financial support from Actelion and Roche/Promedior; grants, personal fees and non-financial support from Boehringer Ingelheim; and personal fees from AstraZeneca, Bayer/MSD, Celgene/Bristol Myers Squibb, Fibrogen, Galapagos, Galecto, Novartis, PureTech, RedX, Sanofi and Shionogi, outside the submitted work.
Figures
Comment in
-
Three wishes for improving clinical drug development in pulmonary fibrosis.Eur Respir J. 2023 Mar 2;61(3):2202355. doi: 10.1183/13993003.02355-2022. Print 2023 Mar. Eur Respir J. 2023. PMID: 36863732 No abstract available.
References
-
- European Medicines Agency . Summary of product characteristics – Esbriet. 2011. www.ema.europa.eu/en/documents/product-information/esbriet-epar-product-... Date last accessed: 7 February 2022.
-
- European Medicines Agency . Summary of product characteristics – Ofev. 2015. www.ema.europa.eu/en/documents/product-information/ofev-epar-product-inf... Date Last accessed: 7 February 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
