Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial
- PMID: 36328372
- PMCID: PMC9631300
- DOI: 10.1136/bmj-2022-071476
Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double blind, placebo controlled trial
Abstract
Objective: To investigate whether oral antimicrobial prophylaxis as an adjunct to intravenous antibiotic prophylaxis reduces surgical site infections after elective colorectal surgery.
Design: Multicentre, randomised, double blind, placebo controlled trial.
Setting: 11 university and non-university hospitals in France between 25 May 2016 and 8 August 2019.
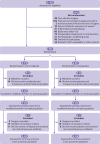
Participants: 926 adults scheduled for elective colorectal surgery.
Intervention: Patients were randomised to receive either a single 1 g dose of ornidazole (n=463) or placebo (n=463) orally 12 hours before surgery, in addition to intravenous antimicrobial prophylaxis before surgical incision.
Main outcome measures: The primary outcome was the proportion of patients with surgical site infection within 30 days after surgery. Secondary outcomes included individual types of surgical site infections and major postoperative complications (Clavien-Dindo classification grade 3 or higher) within 30 days after surgery.
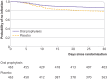
Results: Of the 960 patients who were enrolled, 926 (96%) were included in the analysis. The mean age of participants was 63 years and 554 (60%) were men. Surgical site infection within 30 days after surgery occurred in 60 of 463 patients (13%) in the oral prophylaxis group and 100 of 463 (22%) in the placebo group (absolute difference -8.6%, 95% confidence interval -13.5% to -3.8%; relative risk 0.60, 95% confidence interval 0.45 to 0.80). The proportion of patients with deep infections was 4.8% in the oral prophylaxis group and 8.0% in the placebo group (absolute difference -3.2%, 95% confidence interval -6.4% to -0.1%). The proportion of patients with organ space infections was 5.0% in the oral prophylaxis group and 8.4% in the placebo group (absolute difference -3.4%, -6.7% to -0.2%). Major postoperative complications occurred in 9.1% patients in the oral prophylaxis group and 13.6% in the placebo group (absolute difference -4.5%, -8.6% to -0.5%).
Conclusion: Among adults undergoing elective colorectal surgery, the addition of a single 1 g dose of ornidazole compared with placebo before surgery significantly reduced surgical site infections.
Trial registration: ClinicalTrials.gov NCT02618720.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: the study was funded by a grant from the French Ministry of Health under its Clinical Research Program and by Clermont-Ferrand University Hospital; EF reports receiving consulting fees from Drager Medical, GE Healthcare, and Edwards Lifesciences, and honorariums for presentation from Baxter outside the submitted work; SJ reports receiving consulting fees from Drager Medical, Fisher & Paykel Healthcare, Fresenius Xenios, Medtronic, Baxter, and Mindray outside the submitted work; SL reports receiving consulting fees from Vifor Pharma and Alexys Sante, and honorariums for lectures from Vifor Pharma, Pharmacosmos, Pfizer, and Masimo outside the submitted work; ML reports receiving consulting fees from Gilead, Ambu, and LFB outside the submitted work; AO reports receiving consulting fees from LFB, Orion Pharma, Vifor Pharma, Nordic Pharma, and iSEP, and honorariums for lectures from Orion Pharma, Nordic Pharma, and LFB outside the submitted work. The authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Oral antibiotics before colorectal surgery?BMJ. 2022 Nov 3;379:o2547. doi: 10.1136/bmj.o2547. BMJ. 2022. PMID: 36328356 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
