Perceived feasibility, facilitators and barriers to incorporating point-of-care testing for SARS-CoV-2 into emergency medical services by ambulance service staff: a survey-based approach
- PMID: 36328389
- PMCID: PMC9638752
- DOI: 10.1136/bmjopen-2022-064038
Perceived feasibility, facilitators and barriers to incorporating point-of-care testing for SARS-CoV-2 into emergency medical services by ambulance service staff: a survey-based approach
Abstract
Objectives: This body of work aimed to elicit ambulance service staff's perceptions on the barriers and facilitators to adoption, and clinical utility of incorporating rapid SARS-CoV-2 testing during ambulance assessments.
Design: A mixed-methods survey-based project using a framework analysis method to organise qualitative data.
Setting: Emergency and non-emergency care ambulatory services in the UK were approached to take part.
Participants: Current, practising members of the UK ambulance service (paramedics, technicians, assistants and other staff) were included in this body of work.
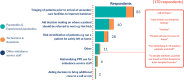
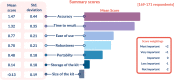
Results: Survey 1: 226 responses were collected between 3 December 2020 and 11 January 2021, 179 (79.2%) of which were completed in full. While the majority of respondents indicated that an ambulance-based testing strategy was feasible in concept (143/190, 75.3%), major barriers to adoption were noted. Many open-ended responses cited concerns regarding misuse of the service by the general public and other healthcare services, timing and conveyance issues, and increased workloads, alongside training and safety concerns. Survey 2: 26 responses were received between 8 February 2021 and 22 February 2021 to this follow-up survey. Survey 2 revealed conveyance decision-making, and risk stratification to be the most frequently prioritised use cases among ambulance service staff. Optimal test characteristics for clinical adoption according to respondents were; accuracy (above 90% sensitivity and specificity), rapidity (<30 min time to results) and ease of sample acquisition.
Conclusions: The majority of commercially available lateral flow devices are unlikely to be supported by paramedics as their duty of care requires both rapid and accurate results that can inform clinical decision making in an emergency situation. Further investigation is needed to define acceptable test characteristics and criteria required for ambulance service staff to be confident and supportive of deployment of a SARS-CoV-2 test in an emergency care setting.
Keywords: COVID-19; Molecular diagnostics; QUALITATIVE RESEARCH.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- FindDX . FindDX SARS-CoV-2 diagnostic pipeline, 2021. Available: https://www.finddx.org/covid-19/pipeline/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
