Pharmacokinetics of hydroxychloroquine in paediatric lupus: data from a novel, direct-to-family clinical trial
- PMID: 36328395
- PMCID: PMC9639143
- DOI: 10.1136/lupus-2022-000811
Pharmacokinetics of hydroxychloroquine in paediatric lupus: data from a novel, direct-to-family clinical trial
Abstract
Objective: Determine the pharmacokinetics (PK) and exposure-response of hydroxychloroquine (HCQ) and desethylhydroxychloroquine (DHCQ) in paediatric SLE (pSLE).
Methods: We conducted an exploratory phase 2, direct-to-family trial. Children enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry with a diagnosis of pSLE were eligible if they were receiving HCQ as standard of care for ≥3 months. Biological samples were collected at up to four visits over a 6-month period. At each visit, plasma was obtained to measure the concentrations of HCQ and DHCQ, as well as cytokines. HCQ and DHCQ plasma PK data were analysed using a population PK modelling approach.
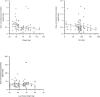
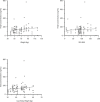
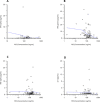
Results: Twenty-five subjects provided a total of 88 plasma concentrations for PK analysis. There was a poor linear fit between HCQ concentrations and total body weight (R2=0.03). There was a decline in both interferon (IFN)-alpha and IFN-gamma with higher concentrations of HCQ and DHCQ. Volume of distribution for HCQ in plasma was higher in children compared with published values in adults (73 000 L vs 44 000 L), but clearance values in children were similar to adults.
Conclusions: We report the first population PK model for HCQ and DHCQ in children using data from a novel direct-to-family clinical trial. We observed high interindividual variability in HCQ PK and found that weight-based dosing for HCQ is poorly correlated with drug concentrations, suggesting the need to use therapeutic drug monitoring to individualise dosing. Furthermore, our results suggest that the current weight-based dosing paradigm for HCQ may result in suboptimal drug exposures, particularly for children with obesity. Accordingly, additional studies of HCQ are needed in pSLE to determine the optimal drug concentration and dosing to reduce disease activity and improve outcomes.
Trial registration number: NCT04358302.
Keywords: interferon type I; pharmacogenetics; pharmacokinetics; systemic lupus erythematosus.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SB receives support from the National Institutes of Health (NIH), the US Food and Drug Administration, the Patient-Centered Outcomes Research Institute, the Rheumatology Research Foundation’s Scientist Development Award, the Childhood Arthritis and Rheumatology Research Alliance (CARRA), Purdue Pharma, and consulting for UCB. RR is supported by the National Institute of General Medical Sciences of the NIH under Award Number T32GM086330. RR’s spouse has current or prior employment and/or stock ownership in financial relationships with Merck & Co and Biogen. DW is an Independent Director for Simulations Plus. LES has received research support from CARRA and Bristol Myers Squib. LES is an Associate Editor for Lupus Science and Medicine and serves on the Data and Safety Monitoring Board for Sanofi (sarilumab) and UCB (certolizumab). Sanofi is a maker of hydroxychloroquine. LES is currently a co-chair of the Registry and Research Oversight Committee for CARRA. She also receives research support from PCORI (PaCr-2017C2-8177) and the CDC (1U01DP006490). CPH receives salary support for research from sponsors for drug development in adults and children (https://dcri.org/about-us/conflict-of-interest/). MC-W receives support for research from the NIH (1U24-MD016258), National Institute of Allergy and Infectious Diseases (HHSN272201500006I, 1K24-AI143971), US Food and Drug Administration (5U18-FD006298), and industry for drug development in adults and children. DG receives salary support for research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD096435 and R01HD102949), and from Nabriva Therapeutics through a contract with the University of North Carolina at Chapel Hill. In addition, DG serves as a consultant for Tellus Therapeutics.
Figures
References
-
- Bertsias GK, Tektonidou M, Amoura Z, et al. . Joint European League against rheumatism and European renal Association–European dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. 10.1136/annrheumdis-2012-201940 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
