Electronic nicotine delivery systems (ENDS) flavours and devices used by adults before and after the 2020 US FDA ENDS enforcement priority: findings from the 2018 and 2020 US ITC Smoking and Vaping Surveys
- PMID: 36328466
- PMCID: PMC9641531
- DOI: 10.1136/tobaccocontrol-2022-057445
Electronic nicotine delivery systems (ENDS) flavours and devices used by adults before and after the 2020 US FDA ENDS enforcement priority: findings from the 2018 and 2020 US ITC Smoking and Vaping Surveys
Abstract
Background: In February 2020, the US Food and Drug Administration (FDA) prioritised enforcement efforts against flavoured prefilled cartridge/pod electronic nicotine delivery systems (ENDS), with the exception of tobacco and menthol. This study examined changes between prepriority enforcement (2018) and early postenforcement (February-June 2020) among adults on: ENDS flavours and devices used most often; location of last purchase of fruit/other-flavoured cartridges (covered under the enforcement priority); and smoking and vaping.
Methods: Prevalence estimates came from 1608 adult frequent (≥weekly) ENDS users (current smokers (n=1072), ex-smokers (n=536)) who participated in the 2018 and/or 2020 US ITC Smoking and Vaping Surveys. Transitions between flavours/devices and changes in smoking/vaping were assessed among baseline respondents who were followed up in 2020 (n=360). Respondents self-reported the ENDS device (disposable, cartridge/pod or tank) and the flavor that they used most often: (1) tobacco flavors (tobacco/tobacco-menthol mix) or unflavored; (2) menthol/mint; (3) fruit/other flavors.
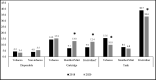
Results: Compared to 2018, in the first 5 months of the 2020 enforcement priority, there were significant increases in the prevalence of fruit/other-flavoured cartridges (7.9% to 12.4%,p=0.026) and menthol/mint cartridges (7.1% to 13.0%, p<0.01) and decreases in tobacco-flavoured tanks (15.5% to 10.0%,p=0.002) and fruit/other-flavoured tanks (38.7% to 33.6%,p=0.038). Fewer than 10% of adults used disposables in 2018 and 2020. Among the cohort sample, the most pronounced transitions between flavours/devices occurred among those who used flavoured cartridges covered under the enforcement priority (54.6% switched to a flavour and/or device excluded from enforcement). There was an increase in purchasing fruit/other-flavoured cartridges online and a decrease in retail locations except for vape shops. Overall, there were few changes in smoking and vaping behaviours.
Conclusions: Between 2018 and the early phase of the FDA's 2020 enforcement priority, prevalence of menthol/mint and fruit/other-flavoured cartridges increased among adults. Half of vapers using cartridge flavours covered in the enforcement switched to other flavours and/or devices that were exempt, with the exception of disposables. The extent to which more comprehensive restrictions may be problematic for adults who prefer a range of ENDS flavours remains uncertain.
Keywords: electronic nicotine delivery devices; nicotine; non-cigarette tobacco products; public policy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KMC has served as paid expert witness in litigation filed against cigarette manufacturers. GTF and DH have served as expert witnesses or consultants on behalf of governments defending their country’s policies or regulations in litigation.
Figures
References
-
- Stastitica . University of Waterloo. e-cigarettes. Available: https://www.statista.com/outlook/cmo/tobacco-products/e-cigarettes/world...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
