Changes in product labelling practices and the use of flavouring chemical additives in vaping products after enactment of statewide flavour legislation
- PMID: 36328467
- PMCID: PMC9664102
- DOI: 10.1136/tc-2022-057469
Changes in product labelling practices and the use of flavouring chemical additives in vaping products after enactment of statewide flavour legislation
Abstract
Introduction: On 18 May 2020, New York State enacted legislation banning the sale of vaping products with distinguishable flavours (other than tobacco). According to this new statute, vaping products are deemed flavoured if they include a statement, whether expressed or implied, that have distinguishable tastes or aromas other than tobacco. This study aimed to determine how manufacturers responded.
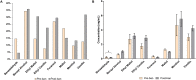
Methods: We collected 555 vaping products from daily vapers (238 preban and 317 postban). We compared preban and postban labelling of products for expressed and implied flavour descriptions, graphics and colours. Flavouring chemicals and concentrations were identified using chromatography methods and were compared preban and postban.
Results: Analysis of the labels preban and postban did not reveal a change in products with expressed flavoured descriptors (45.8% vs 44.2%) and a minimal decrease in implied descriptors (22.3% vs 14.5%). An increase in products without any descriptors was observed (28.2% vs 37.2%) notably within products from a popular pod brand. The average concentration of eight popular flavourings identified preban was 1.4±2.7 compared with 2.3±3.5 mg/mL (p<0.001) postban. No significant changes between individual flavouring concentrations in the most popular refill solutions and pods were found.
Conclusion: While a majority of products appeared to remain non-compliant, this study suggests that enactment of legislation on vaping products making expressed or implied flavour claims may result in some manufacturer changes to product labelling including removal of flavour descriptors. However, use of flavouring additives in vaping products appeared not to be impacted by the ban.
Keywords: Electronic nicotine delivery devices; Non-cigarette tobacco products; Packaging and Labelling; Public policy.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation drugs. Other authors declare no conflict of interest.
Figures


References
-
- Chen JC, Green K, Fryer C, et al. . Perceptions about e-cigarette flavors: a qualitative investigation of young adult cigarette smokers who use e-cigarettes. Addict Res Theory 2019;27:420–8. 10.1080/16066359.2018.1540693 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
