Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx
- PMID: 36328754
- PMCID: PMC9637484
- DOI: 10.1016/j.jare.2022.01.004
Mechanical overloading induces GPX4-regulated chondrocyte ferroptosis in osteoarthritis via Piezo1 channel facilitated calcium influx
Abstract
Introductions: Excessive mechanical stress is closely associated with cell death in various conditions. Exposure of chondrocytes to excessive mechanical loading leads to a catabolic response as well as exaggerated cell death. Ferroptosis is a recently identified form of cell death during cell aging and degeneration. However, it's potential association with mechanical stress remains to be illustrated.
Objectives: To identify whether excessive mechanical stress can cause ferroptosis. To explore the role of mechanical overloading in chondrocyte ferroptosis.
Methods: Chondrocytes were collected from loading and unloading zones of cartilage in patients with osteoarthritis (OA), and the ferroptosis phenotype was analyzed through transmission electron microscope and microarray. Moreover, the relationship between ferroptosis and OA was analyzed by GPX4-conditional knockout (Col2a1-CreERT: GPX4flox/flox) mice OA model and chondrocytes cultured with high strain mechanical stress. Furthermore, the role of Piezo1 ion channel in chondrocyte ferroptosis and OA development was explored by using its inhibitor (GsMTx4) and agonist (Yoda1). Additionally, chondrocyte was cultured in calcium-free medium with mechanical stress, and ferroptosis phenotype was tested.
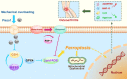
Results: Human cartilage and mouse chondrocyte experiments revealed that mechanical overloading can induce GPX4-associated ferroptosis. Conditional knockout of GPX4 in cartilage aggravated experimental OA process, while additional treatment with ferroptosis suppressor protein (FSP-1) and coenzyme Q10 (CoQ10) abated OA development in GPX4-CKO mice. In mouse OA model and chondrocyte experiments, inhibition of Piezo1 channel activity increased GPX4 expression, attenuated ferroptosis phenotype and reduced the severity of osteoarthritis. Additionally, high strain mechanical stress induced ferroptosis damage in chondrocyte was largely abolished by blocking calcium influx through calcium-free medium.
Conclusions: Our findings show that mechanical overloading induces ferroptosis through Piezo1 activation and subsequent calcium influx in chondrocytes, which might provide a potential target for OA treatment.
Keywords: Chondrocytes; Ferroptosis; Mechanical stress; Osteoarthritis; Piezo1.
Copyright © 2022. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Tang J., Liu C., Li B., Hong S., Li Q., Wang L., et al. Protective Role of Nuclear Factor Erythroid-2-Related Factor 2 against Mechanical Trauma-Induced Apoptosis in a Vaginal Distension-Induced Stress Urinary Incontinence Mouse Model. Oxid Med Cell Longevity. 2019;2019:1–10. doi: 10.1155/2019/2039856. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

