Plasticity of cancer invasion and energy metabolism
- PMID: 36328835
- PMCID: PMC10368441
- DOI: 10.1016/j.tcb.2022.09.009
Plasticity of cancer invasion and energy metabolism
Abstract
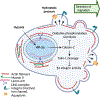
Energy deprivation is a frequent adverse event in tumors that is caused by mutations, malperfusion, hypoxia, and nutrition deficit. The resulting bioenergetic stress leads to signaling and metabolic adaptation responses in tumor cells, secures survival, and adjusts migration activity. The kinetic responses of cancer cells to energy deficit were recently identified, including a switch of invasive cancer cells to energy-conservative amoeboid migration and an enhanced capability for distant metastasis. We review the energy programs employed by different cancer invasion modes including collective, mesenchymal, and amoeboid migration, as well as their interconversion in response to energy deprivation, and we discuss the consequences for metastatic escape. Understanding the energy requirements of amoeboid and other dissemination strategies offers rationales for improving therapeutic targeting of metastatic cancer progression.
Keywords: amoeboid migration; cellular bioenergetics; metabolic stress; migration plasticity.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Mayor R. and Etienne-Manneville S. (2016) The front and rear of collective cell migration. Nat Rev Mol Cell Biol 17, 97–109 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

