JAK2V617F mutation drives vascular resident macrophages toward a pathogenic phenotype and promotes dissecting aortic aneurysm
- PMID: 36329047
- PMCID: PMC9633755
- DOI: 10.1038/s41467-022-34469-1
JAK2V617F mutation drives vascular resident macrophages toward a pathogenic phenotype and promotes dissecting aortic aneurysm
Erratum in
-
Author Correction: JAK2V617F mutation drives vascular resident macrophages toward a pathogenic phenotype and promotes dissecting aortic aneurysm.Nat Commun. 2022 Dec 23;13(1):7921. doi: 10.1038/s41467-022-35645-z. Nat Commun. 2022. PMID: 36564399 Free PMC article. No abstract available.
Abstract
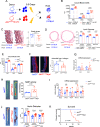
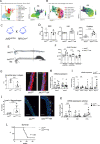
JAK2V617F mutation is associated with an increased risk for athero-thrombotic cardiovascular disease, but its role in aortic disease development and complications remains unknown. In a cohort of patients with myeloproliferative neoplasm, JAK2V617F mutation was identified as an independent risk factor for dilation of both the ascending and descending thoracic aorta. Using single-cell RNA-seq, complementary genetically-modified mouse models, as well as pharmacological approaches, we found that JAK2V617F mutation was associated with a pathogenic pro-inflammatory phenotype of perivascular tissue-resident macrophages, which promoted deleterious aortic wall remodeling at early stages, and dissecting aneurysm through the recruitment of circulating monocytes at later stages. Finally, genetic manipulation of tissue-resident macrophages, or treatment with a Jak2 inhibitor, ruxolitinib, mitigated aortic wall inflammation and reduced aortic dilation and rupture. Overall, JAK2V617F mutation drives vascular resident macrophages toward a pathogenic phenotype and promotes dissecting aortic aneurysm.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

