Stability and degradation in triple cation and methyl ammonium lead iodide perovskite solar cells mediated via Au and Ag electrodes
- PMID: 36329076
- PMCID: PMC9633698
- DOI: 10.1038/s41598-022-19541-6
Stability and degradation in triple cation and methyl ammonium lead iodide perovskite solar cells mediated via Au and Ag electrodes
Abstract
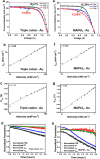
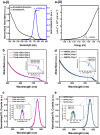
Perovskite solar cells (PSCs), particularly based on the methyl ammonium lead iodide (MAPbI3) formulation, have been of intense interest for the past decade within the photovoltaics (PV) community, given the stupendous rise in power conversion efficiencies (PCEs) attributed to these perovskite formulations, where PCEs have exceeded 25%. However, their long-term stability under operational conditions and environmental storage are still prime challenges to be overcome towards their commercialization. Although studies on the intrinsic perovskite absorber stability have been conducted previously, there are no clear mechanisms for the interaction of electrode-induced absorber degradation pathways, which is the focus of this study. In this report, we have conducted a comprehensive analysis on the impact of the electrode collector layer, specifically Ag and Au, on the degradation mechanism associated with the MAPbI3 and a triple cation absorber, Cs0.05FA0.79MA0.16PbI2.45Br0.55. Notably, Au-based PSCs for both absorbers in an n-i-p architecture showed superior PCE over Ag-based PSCs, where the optimized PCE of MAPbI3 and triple cation-based PSCs was 15.39% and 18.21%, respectively. On the other hand, optimized PCE of MAPbI3 and triple cation-based PSCs with Ag electrodes was 3.02% and 16.44%, respectively. In addition, the Ag-based PSCs showed a rapid decrease in PCE over Au-based PSCs through operational stability measurements. We hypothesize the mechanism of degradation, arising from the Ag interaction with the absorber through the formation of AgI in the PSCs, leads to corrosion of the perovskite absorber, as opposed to the benign AuI when Au electrodes are used in the solar cell stack. Additionally, novel use of photoluminescence spectroscopy (PL) here, allowed us to access key features of the perovskite absorber in situ, while it was in contact with the various layers within the n-i-p solar cell stack. A quenching in the PL peak in the case of Ag-contacted MAPbI3 provided direct evidence of the Ag corrupting the optical properties of the absorber through the formation of AgI which our X-ray diffraction (XRD) results confirmed. This was supported by the fact that an emission peak was still present in the triple cation Ag-device. For the Au-contacted MAPbI3 the presence of a well-defined PL peak, though attenuated from the triple cation Au-device, suggested the AuI does not quell the emission spectrum for either the triple cation or the MAPbI3 absorber. The findings should aid in the understanding and design of new electrode materials with PSCs, which will help accelerate their introduction into the commercial sector in the future.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Stabilizing the Ag Electrode and Reducing J-V Hysteresis through Suppression of Iodide Migration in Perovskite Solar Cells.ACS Appl Mater Interfaces. 2017 Oct 18;9(41):36338-36349. doi: 10.1021/acsami.7b07595. Epub 2017 Oct 6. ACS Appl Mater Interfaces. 2017. PMID: 28952714
-
Enhancing photovoltaic efficiency in Half-Tandem MAPbI3/ MASnI3 Perovskite solar cells with triple core-shell plasmonic nanoparticles.Sci Rep. 2025 Jan 9;15(1):1478. doi: 10.1038/s41598-025-85243-4. Sci Rep. 2025. PMID: 39789094 Free PMC article.
-
Ionic Liquid-Assisted MAPbI3 Nanoparticle-Seeded Growth for Efficient and Stable Perovskite Solar Cells.ACS Appl Mater Interfaces. 2021 May 12;13(18):21194-21206. doi: 10.1021/acsami.1c00677. Epub 2021 Apr 29. ACS Appl Mater Interfaces. 2021. PMID: 33914507
-
Compositional and Interface Engineering of Organic-Inorganic Lead Halide Perovskite Solar Cells.iScience. 2020 Aug 21;23(8):101359. doi: 10.1016/j.isci.2020.101359. Epub 2020 Jul 10. iScience. 2020. PMID: 32712463 Free PMC article. Review.
-
Biomass-Derived Materials in Perovskite Solar Cells: Recent Progress and Future Prospects.Chem Asian J. 2025 Feb 3;20(3):e202401009. doi: 10.1002/asia.202401009. Epub 2024 Nov 29. Chem Asian J. 2025. PMID: 39567262 Review.
Cited by
-
Cs2TiI6 (Cs2TiIxBr6-x) Halide Perovskite Solar Cell and Its Point Defect Analysis.Nanomaterials (Basel). 2023 Jul 19;13(14):2100. doi: 10.3390/nano13142100. Nanomaterials (Basel). 2023. PMID: 37513111 Free PMC article.
-
Strong angular and spectral narrowing of electroluminescence in an integrated Tamm-plasmon-driven halide perovskite LED.Nat Commun. 2024 Jul 10;15(1):5802. doi: 10.1038/s41467-024-49838-1. Nat Commun. 2024. PMID: 38987248 Free PMC article.
-
Hysteresis in Perovskite Devices: Understanding the Abrupt Resistive Switching Mechanism.ACS Energy Lett. 2025 Jul 24;10(8):3983-3992. doi: 10.1021/acsenergylett.5c01556. eCollection 2025 Aug 8. ACS Energy Lett. 2025. PMID: 40808936 Free PMC article.
-
Enhancing the Performance of Perovskite Solar Cells by Introducing 4-(Trifluoromethyl)-1H-imidazole Passivation Agents.Molecules. 2023 Jun 24;28(13):4976. doi: 10.3390/molecules28134976. Molecules. 2023. PMID: 37446637 Free PMC article.
-
High-Performance Perovskite Solar Cells and Modules Fabricated by Slot-Die Coating with Nontoxic Solvents.Nanomaterials (Basel). 2023 May 29;13(11):1760. doi: 10.3390/nano13111760. Nanomaterials (Basel). 2023. PMID: 37299663 Free PMC article.
References
-
- Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009;131:6050–6051. - PubMed
-
- Ashworth C. Reproducible, high-performance perovskite solar cells. Nat. Rev. Mater. 2021;6:293.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

