Systematic evaluation of the pre-eclampsia drugs, dietary supplements and biologicals pipeline using target product profiles
- PMID: 36329468
- PMCID: PMC9635102
- DOI: 10.1186/s12916-022-02582-z
Systematic evaluation of the pre-eclampsia drugs, dietary supplements and biologicals pipeline using target product profiles
Abstract
Background: The Accelerating Innovation for Mothers (AIM) project established a database of candidate medicines in research and development (R&D) between 2000 and 2021 for five pregnancy-related conditions, including pre-eclampsia. In parallel, we published target product profiles (TPPs) that describe optimal characteristics of medicines for use in preventing/treating pre-eclampsia. The study objective was to use systematic double screening and extraction to identify all candidate medicines being investigated for pre-eclampsia prevention/treatment and rank their potential based on the TPPs.
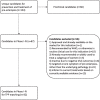
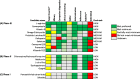
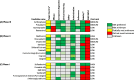
Methods: Adis Insight, Pharmaprojects, WHO international clinical trials registry platform (ICTRP), PubMed and grant databases were searched (Jan-May 2021). The AIM database was screened for all candidates being investigated for pre-eclampsia. Candidates in clinical development were evaluated against nine prespecified criteria from TPPs identified as key for wide-scale implementation, and classified as high, medium or low potential based on matching to the TPPs. Preclinical candidates were categorised by product type, archetype and medicine subclass.
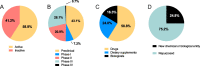
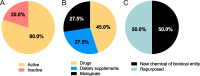
Results: The AIM database identified 153 candidates for pre-eclampsia. Of the 87 candidates in clinical development, seven were classified as high potential (prevention: esomeprazole, L-arginine, chloroquine, vitamin D and metformin; treatment: sulfasalazine and metformin) and eight as medium potential (prevention: probiotic lactobacilli, dalteparin, selenium and omega-3 fatty acid; treatment: sulforaphane, pravastatin, rosuvastatin and vitamin B3). Sixty-six candidates were in preclinical development, the most common being amino acid/peptides, siRNA-based medicines and polyphenols.
Conclusions: This is a novel, evidence-informed approach to identifying promising candidates for pre-eclampsia prevention and treatment - a vital step in stimulating R&D of new medicines for pre-eclampsia suitable for real-world implementation.
Keywords: Chloroquine; Drug development; Esomeprazole; Hypertension; L-Arginine; Maternal medicine; Metformin; Pregnancy; Sulfasalazine; Vitamin D.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
New medicines for spontaneous preterm birth prevention and preterm labour management: landscape analysis of the medicine development pipeline.BMC Pregnancy Childbirth. 2023 Jul 18;23(1):525. doi: 10.1186/s12884-023-05842-9. BMC Pregnancy Childbirth. 2023. PMID: 37464260 Free PMC article.
-
Analysis of a maternal health medicines pipeline database 2000-2021: New candidates for the prevention and treatment of fetal growth restriction.BJOG. 2023 May;130(6):653-663. doi: 10.1111/1471-0528.17392. Epub 2023 Feb 5. BJOG. 2023. PMID: 36655375
-
Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia.Am J Obstet Gynecol. 2022 Feb;226(2S):S1157-S1170. doi: 10.1016/j.ajog.2020.09.014. Epub 2020 Sep 16. Am J Obstet Gynecol. 2022. PMID: 32946849
-
Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems.Cochrane Database Syst Rev. 2018 Oct 1;10(10):CD001059. doi: 10.1002/14651858.CD001059.pub5. Cochrane Database Syst Rev. 2018. PMID: 30277579 Free PMC article.
-
Innovations in the prevention and treatment of postpartum hemorrhage: Analysis of a novel medicines development pipeline database.Int J Gynaecol Obstet. 2022 Jun;158 Suppl 1(Suppl 1):31-39. doi: 10.1002/ijgo.14200. Int J Gynaecol Obstet. 2022. PMID: 35762804 Free PMC article.
Cited by
-
Secondary prevention of preeclampsia.Front Cell Dev Biol. 2025 Feb 7;13:1520218. doi: 10.3389/fcell.2025.1520218. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 39989985 Free PMC article. Review.
-
Factors Influencing Pregnant Women's Participation in Randomised Clinical Trials in India: A Qualitative Study.BJOG. 2025 May;132(6):772-781. doi: 10.1111/1471-0528.18074. Epub 2025 Jan 28. BJOG. 2025. PMID: 39871821 Free PMC article.
-
Multimodal ultrasound-based radiomics and deep learning for differential diagnosis of O-RADS 4-5 adnexal masses.Cancer Imaging. 2025 May 23;25(1):64. doi: 10.1186/s40644-025-00883-z. Cancer Imaging. 2025. PMID: 40410823 Free PMC article.
-
Statins for preventing preeclampsia.Cochrane Database Syst Rev. 2025 Mar 18;3(3):CD016133. doi: 10.1002/14651858.CD016133. Cochrane Database Syst Rev. 2025. PMID: 40099754 Free PMC article.
-
Polyphenols for the Prevention or Management of Preeclampsia: A Systematic Review and Meta-Analysis.BJOG. 2025 Jun;132(7):867-879. doi: 10.1111/1471-0528.18106. Epub 2025 Mar 3. BJOG. 2025. PMID: 40025969 Free PMC article.
References
-
- WHO. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva; 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

