Assessing the genetic burden of familial hypercholesterolemia in a large middle eastern biobank
- PMID: 36329474
- PMCID: PMC9635206
- DOI: 10.1186/s12967-022-03697-w
Assessing the genetic burden of familial hypercholesterolemia in a large middle eastern biobank
Abstract
Background: The genetic architecture underlying Familial Hypercholesterolemia (FH) in Middle Eastern Arabs is yet to be fully described, and approaches to assess this from population-wide biobanks are important for public health planning and personalized medicine.
Methods: We evaluate the pilot phase cohort (n = 6,140 adults) of the Qatar Biobank (QBB) for FH using the Dutch Lipid Clinic Network (DLCN) criteria, followed by an in-depth characterization of all genetic alleles in known dominant (LDLR, APOB, and PCSK9) and recessive (LDLRAP1, ABCG5, ABCG8, and LIPA) FH-causing genes derived from whole-genome sequencing (WGS). We also investigate the utility of a globally established 12-SNP polygenic risk score to predict FH individuals in this cohort with Arab ancestry.
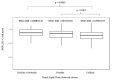
Results: Using DLCN criteria, we identify eight (0.1%) 'definite', 41 (0.7%) 'probable' and 334 (5.4%) 'possible' FH individuals, estimating a prevalence of 'definite or probable' FH in the Qatari cohort of ~ 1:125. We identify ten previously known pathogenic single-nucleotide variants (SNVs) and 14 putatively novel SNVs, as well as one novel copy number variant in PCSK9. Further, despite the modest sample size, we identify one homozygote for a known pathogenic variant (ABCG8, p. Gly574Arg, global MAF = 4.49E-05) associated with Sitosterolemia 2. Finally, calculation of polygenic risk scores found that individuals with 'definite or probable' FH have a significantly higher LDL-C SNP score than 'unlikely' individuals (p = 0.0003), demonstrating its utility in Arab populations.
Conclusion: We design and implement a standardized approach to phenotyping a population biobank for FH risk followed by systematically identifying known variants and assessing putative novel variants contributing to FH burden in Qatar. Our results motivate similar studies in population-level biobanks - especially those with globally under-represented ancestries - and highlight the importance of genetic screening programs for early detection and management of individuals with high FH risk in health systems.
Keywords: Cholesterol; Dutch lipid Clinic Network; Dyslipidemias; LDL; LDLRAP1; Lipoproteins/Receptors; Middle East region.; Polygenic risk scores; Premature coronary artery disease; Sitosterolemia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J. 2016;37(17):1384–94. - PubMed
-
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, National Lipid Association Expert Panel on Familial H Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):9–17. - PubMed
-
- Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168(1):1–14. - PubMed
-
- Sjouke B, Kusters DM, Kindt I, Besseling J, Defesche JC, Sijbrands EJ, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36(9):560–5. - PubMed
-
- Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132(22):2167–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

