Environmental enrichment reverses cerebellar impairments caused by prenatal exposure to a synthetic glucocorticoid
- PMID: 36329900
- PMCID: PMC9581733
- DOI: 10.3934/Neuroscience.2022018
Environmental enrichment reverses cerebellar impairments caused by prenatal exposure to a synthetic glucocorticoid
Abstract
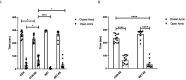
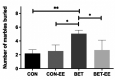
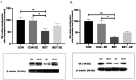
During prenatal life, exposure to synthetic glucocorticoids (SGCs) can alter normal foetal development, resulting in disease later in life. Previously, we have shown alterations in the dendritic cytoarchitecture of Purkinje cells in adolescent rat progeny prenatally exposed to glucocorticoids. However, the molecular mechanisms underlying these alterations remain unclear. A possible molecular candidate whose deregulation may underlie these changes is the glucocorticoid receptor (GR) and neurotrophin 3/ tropomyosin receptor kinase C, neurotrophic complex (NT-3/TrkC), which specifically modulates the development of the neuronal connections in the cerebellar vermis. To date, no evidence has shown that the cerebellar expression levels of this neurotrophic complex are affected by exposure to a synthetic glucocorticoid in utero. Therefore, the first objective of this investigation was to evaluate the expression of GR, NT-3 and TrkC in the cerebellar vermis using immunohistochemistry and western blot techniques by evaluating the progeny during the postnatal stage equivalent to adolescence (postnatal Day 52). Additionally, we evaluated anxiety-like behaviours in progeny using the elevated plus maze and the marble burying test. In addition, an environmental enrichment (EE) can increase the expression of some neurotrophins and has anxiolytic power. Therefore, we wanted to assess whether an EE reversed the long-term alterations induced by prenatal betamethasone exposure. The major findings of this study were as follows: i) prenatal betamethasone (BET) administration decreases GR, NT-3 and TrkC expression in the cerebellar vermis ii) prenatal BET administration decreases GR expression in the cerebellar hemispheres and iii) enhances the anxiety-like behaviours in the same progeny, and iv) exposure to an EE reverses the reduced expression of GR, NT-3 and TrkC in the cerebellar vermis and v) decreases anxiety-like behaviours. In conclusion, an enriched environment applied 18 days post-weaning was able to restabilize GR, NT-3 and TrkC expression levels and reverse anxious behaviours observed in adolescent rats prenatally exposed to betamethasone.
Keywords: GR; NT-3; TrkC; anxiety-like behaviours; betamethasone; environmental enrichment.
© 2022 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures







Similar articles
-
Environmental enrichment restores the reduced expression of cerebellar synaptophysin and the motor coordination impairment in rats prenatally treated with betamethasone.Physiol Behav. 2019 Oct 1;209:112590. doi: 10.1016/j.physbeh.2019.112590. Epub 2019 Jun 25. Physiol Behav. 2019. PMID: 31252027
-
Antenatal betamethasone produces protracted changes in anxiety-like behaviors and in the expression of microtubule-associated protein 2, brain-derived neurotrophic factor and the tyrosine kinase B receptor in the rat cerebellar cortex.Int J Dev Neurosci. 2015 Jun;43:78-85. doi: 10.1016/j.ijdevneu.2015.04.005. Epub 2015 Apr 15. Int J Dev Neurosci. 2015. PMID: 25889225
-
Environmental enrichment alters neurotrophin levels after fetal alcohol exposure in rats.Alcohol Clin Exp Res. 2008 Oct;32(10):1741-51. doi: 10.1111/j.1530-0277.2008.00759.x. Epub 2008 Jul 24. Alcohol Clin Exp Res. 2008. PMID: 18652597 Free PMC article.
-
Constitutive phosphorylation of TrkC receptors in cultured cerebellar granule neurons might be responsible for the inability of NT-3 to increase neuronal survival and to activate p21 Ras.Neurochem Res. 1996 Jul;21(7):851-9. doi: 10.1007/BF02532309. Neurochem Res. 1996. PMID: 8873090
-
Neurotrophin-3 in the development of the enteric nervous system.Prog Brain Res. 2004;146:243-63. doi: 10.1016/S0079-6123(03)46016-0. Prog Brain Res. 2004. PMID: 14699968 Review.
Cited by
-
[Effects of antenatal corticosteroids on outcomes of very premature infants and neurodevelopment during infancy].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Oct 15;26(10):1040-1045. doi: 10.7499/j.issn.1008-8830.2405073. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 39467672 Free PMC article. Chinese.
References
LinkOut - more resources
Full Text Sources
Research Materials
