Chronic treatment with escitalopram and venlafaxine affects the neuropeptide S pathway differently in adult Wistar rats exposed to maternal separation
- PMID: 36329901
- PMCID: PMC9581731
- DOI: 10.3934/Neuroscience.2022022
Chronic treatment with escitalopram and venlafaxine affects the neuropeptide S pathway differently in adult Wistar rats exposed to maternal separation
Abstract
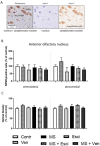
Neuropeptide S (NPS), which is a peptide that is involved in the regulation of the stress response, seems to be relevant to the mechanism of action of antidepressants that have anxiolytic properties. However, to date, there have been no reports regarding the effect of long-term treatment with escitalopram or venlafaxine on the NPS system under stress conditions. This study aimed to investigate the effects of the above-mentioned antidepressants on the NPS system in adult male Wistar rats that were exposed to neonatal maternal separation (MS). Animals were exposed to MS for 360 min. on postnatal days (PNDs) 2-15. MS causes long-lasting behavioral, endocrine and neurochemical consequences that mimic anxiety- and depression-related features. MS and non-stressed rats were given escitalopram or venlafaxine (10mg/kg) IP from PND 69 to 89. The NPS system was analyzed in the brainstem, hypothalamus, amygdala and anterior olfactory nucleus using quantitative RT-PCR and immunohistochemical methods. The NPS system was vulnerable to MS in the brainstem and amygdala. In the brainstem, escitalopram down-regulated NPS and NPS mRNA in the MS rats and induced a tendency to reduce the number of NPS-positive cells in the peri-locus coeruleus. In the MS rats, venlafaxine insignificantly decreased the NPSR mRNA levels in the amygdala and a number of NPSR cells in the basolateral amygdala, and increased the NPS mRNA levels in the hypothalamus. Our data show that the studied antidepressants affect the NPS system differently and preliminarily suggest that the NPS system might partially mediate the pharmacological effects that are induced by these drugs.
Keywords: antidepressants; maternal separation; neuropeptide S; neuropeptide S receptor.
© 2022 the Author(s), licensee AIMS Press.
Conflict of interest statement
Conflict of interest: The authors do not declare any conflict of interest.
Figures







Similar articles
-
Escitalopram alters the hypothalamic OX system but does not affect its up-regulation induced by early-life stress in adult rats.Neurosci Res. 2022 Jul;180:58-71. doi: 10.1016/j.neures.2022.02.005. Epub 2022 Feb 25. Neurosci Res. 2022. PMID: 35219722
-
Modulatory effect of long-term treatment with escitalopram and clonazepam on the expression of anxiety-related neuropeptides: neuromedin U, neuropeptide S and their receptors in the rat brain.Mol Biol Rep. 2022 Sep;49(9):9041-9049. doi: 10.1007/s11033-022-07578-9. Epub 2022 Jun 11. Mol Biol Rep. 2022. PMID: 35690686
-
Escitalopram alters local expression of noncanonical stress-related neuropeptides in the rat brain via NPS receptor signaling.Pharmacol Rep. 2022 Aug;74(4):637-653. doi: 10.1007/s43440-022-00374-z. Epub 2022 Jun 2. Pharmacol Rep. 2022. PMID: 35653031
-
Neuropeptide S as a novel arousal promoting peptide transmitter.FEBS J. 2005 Nov;272(22):5689-93. doi: 10.1111/j.1742-4658.2005.04982.x. FEBS J. 2005. PMID: 16279934 Review.
-
Pathophysiological and therapeutic implications of neuropeptide S system in neurological disorders.Peptides. 2024 May;175:171167. doi: 10.1016/j.peptides.2024.171167. Epub 2024 Feb 6. Peptides. 2024. PMID: 38325715 Review.
References
LinkOut - more resources
Full Text Sources
