Clinical outcomes of subcutaneous vs. transvenous implantable defibrillator therapy in a polymorbid patient cohort
- PMID: 36330004
- PMCID: PMC9624387
- DOI: 10.3389/fcvm.2022.1008311
Clinical outcomes of subcutaneous vs. transvenous implantable defibrillator therapy in a polymorbid patient cohort
Abstract
Background: The subcutaneous implantable cardioverter-defibrillator (S-ICD) has been designed to overcome lead-related complications and device endocarditis. Lacking the ability for pacing or resynchronization therapy its usage is limited to selected patients at risk for sudden cardiac death (SCD).
Objective: The aim of this single-center study was to assess clinical outcomes of S-ICD and single-chamber transvenous (TV)-ICD in an all-comers population.
Methods: The study cohort comprised a total of 119 ICD patients who underwent either S-ICD (n = 35) or TV-ICD (n = 84) implantation at the University Hospital Frankfurt from 2009 to 2017. By applying an inverse probability-weighting (IPW) analysis based on the propensity score including the Charlson Comorbidity Index (CCI) to adjust for potential extracardiac comorbidities, we aimed for head-to-head comparison on the study composite endpoint: overall survival, hospitalization, and device-associated events (including appropriate and inappropriate shocks or system-related complications).
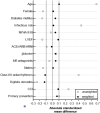
Results: The median age of the study population was 66.0 years, 22.7% of the patients were female. The underlying heart disease was ischemic cardiomyopathy (61.4%) with a median LVEF of 30%. Only 52.9% had received an ICD for primary prevention, most of the patients (67.3%) had advanced heart failure (NYHA class II-III) and 16.8% were in atrial fibrillation. CCI was 5 points in TV-ICD patients vs. 4 points for patients with S-ICD (p = 0.209) indicating increased morbidity. The composite endpoint occurred in 38 patients (31.9 %), revealing no significant difference between patients implanted with an S-ICD or TV-ICD (unweighted HR 1.50, 95 % confidence interval (CI) 0.78-2.90; p = 0.229, weighted HR 0.94, 95% CI, 0.61-1.50, p = 0.777). Furthermore, we observed no difference in any single clinical endpoint or device-associated outcome, neither in the unweighted cohort nor following inverse probability-weighting.
Conclusion: Clinical outcomes of the S-ICD and TV-ICD revealed no differences in the composite endpoint including survival, freedom of hospitalization and device-associated events, even after careful adjustment for potential confounders. Moreover, the CCI was evaluated in a S-ICD cohort demonstrating higher survival rates than predicted by the CCI in young, polymorbid (S-)ICD patients.
Keywords: S-ICD; TV-ICD; implantable cardioverter-defibrillator (ICD); subcutaneous ICD; sudden cardiac death; transvenous ICD.
Copyright © 2022 Kattih, Operhalski, Boeckling, Hecker, Michael, Vamos, Hohnloser and Erath.
Conflict of interest statement
Author JE reports receiving consultant fees, travel support and lecture fees from ZOLL Medical, travel grants from Bayer Vital, St. Jude Medical/Abbott, Novartis and lecture fees from Servier, Pfizer and Bayer Vital and was a fellow of the Boston Scientific heart rhythm fellowship program. Author MV reports consulting fees and/or non-financial support from Biotronik, Medtronic, and Pfizer, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter automatic defibrillator implantation trial investigator. N Engl J Med. (1996) 335:1933–40. 10.1056/NEJM199612263352601 - DOI - PubMed
-
- Tan VH, Wilton SB, Kuriachan V, Sumner GL, Exner DV. Impact of programming strategies aimed at reducing nonessential implantable cardioverter defibrillator therapies on mortality: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. (2014) 7:164–70. 10.1161/CIRCEP.113.001217 - DOI - PubMed
LinkOut - more resources
Full Text Sources

