Effective specialist or jack of all trades? Experimental evolution of a crop pest in fluctuating and stable environments
- PMID: 36330306
- PMCID: PMC9624081
- DOI: 10.1111/eva.13360
Effective specialist or jack of all trades? Experimental evolution of a crop pest in fluctuating and stable environments
Abstract
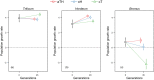
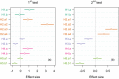
Understanding pest evolution in agricultural systems is crucial for developing effective and innovative pest control strategies. Types of cultivation, such as crop monocultures versus polycultures or crop rotation, may act as a selective pressure on pests' capability to exploit the host's resources. In this study, we examined the herbivorous mite Aceria tosichella (commonly known as wheat curl mite), a widespread wheat pest, to understand how fluctuating versus stable environments influence its niche breadth and ability to utilize different host plant species. We subjected a wheat-bred mite population to replicated experimental evolution in a single-host environment (either wheat or barley), or in an alternation between these two plant species every three mite generations. Next, we tested the fitness of these evolving populations on wheat, barley, and on two other plant species not encountered during experimental evolution, namely rye and smooth brome. Our results revealed that the niche breadth of A. tosichella evolved in response to the level of environmental variability. The fluctuating environment expanded the niche breadth by increasing the mite's ability to utilize different plant species, including novel ones. Such an environment may thus promote flexible host-use generalist phenotypes. However, the niche expansion resulted in some costs expressed as reduced performances on both wheat and barley as compared to specialists. Stable host environments led to specialized phenotypes. The population that evolved in a constant environment consisting of barley increased its fitness on barley without the cost of utilizing wheat. However, the population evolving on wheat did not significantly increase its fitness on wheat, but decreased its performance on barley. Altogether, our results indicated that, depending on the degree of environmental heterogeneity, agricultural systems create different conditions that influence pests' niche breadth evolution, which may in turn affect the ability of pests to persist in such systems.
Keywords: Aceria tosichella; environmental variability; experimental evolution; host range; niche breadth; wheat curl mite.
© 2022 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Altieri, M. A. (1999). The ecological role of biodiversity in agroecosystems. Agriculture, Ecosystems & Environment, 74(1–3), 19–31. 10.1016/S0167-8809(99)00028-6 - DOI
-
- Andow, D. A. (1991). Vegetational diversity and arthropod population response. Annual Review of Entomology, 36(1), 561–586. 10.1146/annurev.en.36.010191.003021 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

