Neuroimaging features in inflammatory myelopathies: A review
- PMID: 36330423
- PMCID: PMC9623025
- DOI: 10.3389/fneur.2022.993645
Neuroimaging features in inflammatory myelopathies: A review
Abstract
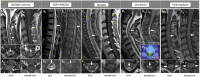
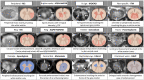
Spinal cord involvement can be observed in the course of immune-mediated disorders. Although multiple sclerosis (MS) represents the leading cause of inflammatory myelopathy, an increasing number of alternative etiologies must be now considered in the diagnostic work-up of patients presenting with myelitis. These include antibody-mediated disorders and cytotoxic T cell-mediated diseases targeting central nervous system (CNS) antigens, and systemic autoimmune conditions with secondary CNS involvement. Even though clinical features are helpful to orient the diagnostic suspicion (e.g., timing and severity of myelopathy symptoms), the differential diagnosis of inflammatory myelopathies is often challenging due to overlapping features. Moreover, noninflammatory etiologies can sometimes mimic an inflammatory process. In this setting, magnetic resonance imaging (MRI) is becoming a fundamental tool for the characterization of spinal cord damage, revealing a pictorial scenario which is wider than the clinical manifestations. The characterization of spinal cord lesions in terms of longitudinal extension, location on axial plane, involvement of the white matter and/or gray matter, and specific patterns of contrast enhancement, often allows a proper differentiation of these diseases. For instance, besides classical features, such as the presence of longitudinally extensive spinal cord lesions in patients with aquaporin-4-IgG positive neuromyelitis optica spectrum disorder (AQP4+NMOSD), novel radiological signs (e.g., H sign, trident sign) have been recently proposed and successfully applied for the differential diagnosis of inflammatory myelopathies. In this review article, we will discuss the radiological features of spinal cord involvement in autoimmune disorders such as MS, AQP4+NMOSD, myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), and other recently characterized immune-mediated diseases. The identification of imaging pitfalls and mimics that can lead to misdiagnosis will also be examined. Since spinal cord damage is a major cause of irreversible clinical disability, the recognition of these radiological aspects will help clinicians achieve a correct and prompt diagnosis, treat early with disease-specific treatment and improve patient outcomes.
Keywords: MRI; multiple sclerosis; myelin oligodendrocyte glycoprotein; myelopathies; neuromyelitis optica spectrum disorders; paraneoplastic; transverse myelitis.
Copyright © 2022 Cacciaguerra, Sechi, Rocca, Filippi, Pittock and Flanagan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

