Arginase-1 targeting peptide vaccine in patients with metastatic solid tumors - A phase I trial
- PMID: 36330525
- PMCID: PMC9622376
- DOI: 10.3389/fimmu.2022.1023023
Arginase-1 targeting peptide vaccine in patients with metastatic solid tumors - A phase I trial
Abstract
Background: Arginase-1-producing cells inhibit T cell-mediated anti-tumor responses by reducing L-arginine levels in the tumor microenvironment. T cell-facilitated elimination of arginase-1-expressing cells could potentially restore L-arginine levels and improve anti-tumor responses. The activation of arginase-1-specific T cells may convert the immunosuppressive tumor microenvironment and induce or strengthen local Th1 inflammation. In the current clinical study, we examined the safety and immunogenicity of arginase-1-based peptide vaccination.
Methods: In this clinical phase I trial, ten patients with treatment-refractory progressive solid tumors were treated. The patients received an arginase-1 peptide vaccine comprising three 20-mer peptides from the ARG1 immunological "hot spot" region in combination with the adjuvant Montanide ISA-51. The vaccines were administered subcutaneously every third week (maximum 16 vaccines). The primary endpoint was to evaluate safety assessed by Common Terminology Criteria for Adverse Events 4.0 and laboratory monitoring. Vaccine-specific immune responses were evaluated using enzyme-linked immune absorbent spot assays and intracellular cytokine staining on peripheral blood mononuclear cells. Clinical responses were evaluated using Response Evaluation Criteria in Solid Tumors 1.1.
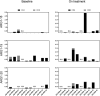
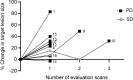
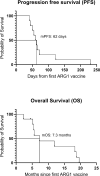
Results: The vaccination was feasible, and no vaccine-related grade 3-4 adverse events were registered. Nine (90%) of ten patients exhibited peptide-specific immune responses in peripheral blood mononuclear cells. Six (86%) of the seven evaluable patients developed a reactive T cell response against at least one of the ARG1 peptides during treatment. A phenotypic classification revealed that arginase-1 vaccine-specific T cells were both CD4+ T cells and CD8+ T cells. Two (20%) of ten patients obtained stable disease for respectively four- and seven months on vaccination treatment.
Conclusion: The peptide vaccine against arginase-1 was safe. Nine (90%) of ten patients had measurable peptide-specific responses in the periphery blood, and two (20%) of ten patients attained stable disease on protocol treatment.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT03689192, identifier NCT03689192.
Keywords: arginase-1; first-in-human; peptide; solid tumors; vaccination.
Copyright © 2022 Lorentzen, Martinenaite, Kjeldsen, Holmstroem, Mørk, Pedersen, Ehrnrooth, Andersen and Svane.
Conflict of interest statement
MA has various patent applications in relation to the therapeutic uses of ARG1 peptides. The patents are allocated to the company IO Biotech. MA is a founder, advisor, and shareholder for IO Biotech. EM, AP, and EE are employees at IO Biotech. IS has lectured for or had advisory board relationships with MSD, Sanofi Aventis, BMS, Pierre Fabre, Novartis, TILT Biotherapeutics, IO Biotech, and Novo Nordisk. IS has received research grants from Lytix biopharma, IO Biotech, BMS, Adaptimmune, and TILT Biotherapeutics. IS is a co-founder and shareholder for the company IO Biotech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

