Adenovirus vector and mRNA vaccines: Mechanisms regulating their immunogenicity
- PMID: 36330560
- PMCID: PMC9877955
- DOI: 10.1002/eji.202250022
Adenovirus vector and mRNA vaccines: Mechanisms regulating their immunogenicity
Abstract
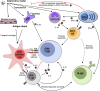
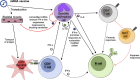
Replication-incompetent adenovirus (Ad) vector and mRNA-lipid nanoparticle (LNP) constructs represent two modular vaccine platforms that have attracted substantial interest over the past two decades. Due to the COVID-19 pandemic and the rapid development of multiple successful vaccines based on these technologies, there is now clear real-world evidence of the utility and efficacy of these platforms. Considerable optimization and refinement efforts underpin the successful application of these technologies. Despite this, our understanding of the specific pathways and processes engaged by these vaccines to stimulate the immune response remains incomplete. This review will synthesize our current knowledge of the specific mechanisms by which CD8+ T cell and antibody responses are induced by each of these vaccine platforms, and how this can be impacted by specific vaccine construction techniques. Key gaps in our knowledge are also highlighted, which can hopefully focus future studies.
Keywords: COVID-19; adenovirus vector; cellular memory; innate response; mRNA vaccines.
© 2022 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
P.K. is a named inventor on a patent application in the field of cancer vaccines. N.P. declares no commercial or financial conflict of interest.
Figures
References
-
- WHO . Status of COVID‐19 Vaccines within WHO EUL/PQ evaluation process. World Health Organization; 2022. Available at: https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVI...
-
- Ishola, D. , Manno, D. , Afolabi, M. O. , Keshinro, B. , Bockstal, V. , Rogers, B. , Owusu‐Kyei, K. et al., Safety and long‐term immunogenicity of the two‐dose heterologous Ad26.ZEBOV and MVA‐BN‐Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open‐label, non‐randomised stage 1, and a randomised, double‐blind, controlled stage 2 trial. Lancet Infect. Dis. 2022. 22: 97–109. - PMC - PubMed
-
- Xiang, Z. Q. , Yang, Y. , Wilson, J. M. and Ertl, H. C. , A replication‐defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996. 219: 220–227. - PubMed
-
- Martinon, F. , Krishnan, S. , Lenzen, G. , Magné, R. , Gomard, E. , Guillet, J. , Lévy, J. et al., Induction of virus‐specific cytotoxic T lymphocytes in vivo by liposome‐entrapped mRNA. Eur. J. Immunol. 1993. 23: 1719–1722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

