Cardiomyocyte BRAF is a key signalling intermediate in cardiac hypertrophy in mice
- PMID: 36331065
- PMCID: PMC9679367
- DOI: 10.1042/CS20220607
Cardiomyocyte BRAF is a key signalling intermediate in cardiac hypertrophy in mice
Abstract
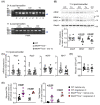
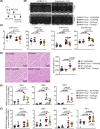
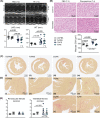
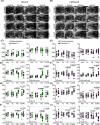
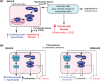
Cardiac hypertrophy is necessary for the heart to accommodate an increase in workload. Physiological, compensated hypertrophy (e.g. with exercise) is reversible and largely due to cardiomyocyte hypertrophy. Pathological hypertrophy (e.g. with hypertension) is associated with additional features including increased fibrosis and can lead to heart failure. RAF kinases (ARAF/BRAF/RAF1) integrate signals into the extracellular signal-regulated kinase 1/2 cascade, a pathway implicated in cardiac hypertrophy, and activation of BRAF in cardiomyocytes promotes compensated hypertrophy. Here, we used mice with tamoxifen-inducible cardiomyocyte-specific BRAF knockout (CM-BRAFKO) to assess the role of BRAF in hypertension-associated cardiac hypertrophy induced by angiotensin II (AngII; 0.8 mg/kg/d, 7 d) and physiological hypertrophy induced by phenylephrine (40 mg/kg/d, 7 d). Cardiac dimensions/functions were measured by echocardiography with histological assessment of cellular changes. AngII promoted cardiomyocyte hypertrophy and increased fibrosis within the myocardium (interstitial) and around the arterioles (perivascular) in male mice; cardiomyocyte hypertrophy and interstitial (but not perivascular) fibrosis were inhibited in mice with CM-BRAFKO. Phenylephrine had a limited effect on fibrosis but promoted cardiomyocyte hypertrophy and increased contractility in male mice; cardiomyocyte hypertrophy was unaffected in mice with CM-BRAFKO, but the increase in contractility was suppressed and fibrosis increased. Phenylephrine induced a modest hypertrophic response in female mice and, in contrast with the males, tamoxifen-induced loss of cardiomyocyte BRAF reduced cardiomyocyte size, had no effect on fibrosis and increased contractility. The data identify BRAF as a key signalling intermediate in both physiological and pathological hypertrophy in male mice, and highlight the need for independent assessment of gene function in females.
Keywords: BRAF; cardiac hypertrophy; cardiomyocytes; fibrosis; hypertension; protein-serine-threonine kinases.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures




References
-
- Tarone G., Balligand J.L., Bauersachs J., Clerk A., de Windt L., Heymans S.et al. (2014) Targeting myocardial remodelling to develop novel therapies for heart failure: A position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur. J. Heart Fail. 16, 494–508 10.1002/ejhf.62 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

