Targeting UGCG Overcomes Resistance to Lysosomal Autophagy Inhibition
- PMID: 36331284
- PMCID: PMC9905280
- DOI: 10.1158/2159-8290.CD-22-0535
Targeting UGCG Overcomes Resistance to Lysosomal Autophagy Inhibition
Abstract
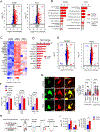
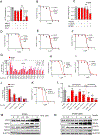
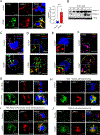
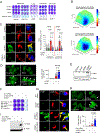
Lysosomal autophagy inhibition (LAI) with hydroxychloroquine or DC661 can enhance cancer therapy, but tumor regrowth is common. To elucidate LAI resistance, proteomics and immunoblotting demonstrated that LAI induced lipid metabolism enzymes in multiple cancer cell lines. Lipidomics showed that LAI increased cholesterol, sphingolipids, and glycosphingolipids. These changes were associated with striking levels of GM1+ membrane microdomains (GMM) in plasma membranes and lysosomes. Inhibition of cholesterol/sphingolipid metabolism proteins enhanced LAI cytotoxicity. Targeting UDP-glucose ceramide glucosyltransferase (UGCG) synergistically augmented LAI cytotoxicity. Although UGCG inhibition decreased LAI-induced GMM and augmented cell death, UGCG overexpression led to LAI resistance. Melanoma patients with high UGCG expression had significantly shorter disease-specific survival. The FDA-approved UGCG inhibitor eliglustat combined with LAI significantly inhibited tumor growth and improved survival in syngeneic tumors and a therapy-resistant patient-derived xenograft. These findings nominate UGCG as a new cancer target, and clinical trials testing UGCG inhibition in combination with LAI are warranted.
Significance: We discovered UGCG-dependent lipid remodeling drives resistance to LAI. Targeting UGCG with a drug approved for a lysosomal storage disorder enhanced LAI antitumor activity without toxicity. LAI and UGCG inhibition could be tested clinically in multiple cancers. This article is highlighted in the In This Issue feature, p. 247.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

