The X factor in neurodegeneration
- PMID: 36331399
- PMCID: PMC9641640
- DOI: 10.1084/jem.20211488
The X factor in neurodegeneration
Abstract
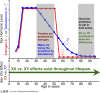
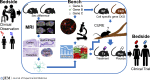
Given the aging population, it is important to better understand neurodegeneration in aging healthy people and to address the increasing incidence of neurodegenerative diseases. It is imperative to apply novel strategies to identify neuroprotective therapeutics. The study of sex differences in neurodegeneration can reveal new candidate treatment targets tailored for women and men. Sex chromosome effects on neurodegeneration remain understudied and represent a promising frontier for discovery. Here, we will review sex differences in neurodegeneration, focusing on the study of sex chromosome effects in the context of declining levels of sex hormones during aging.
© 2022 Voskuhl and Itoh.
Conflict of interest statement
Disclosures: R. Voskuhl reported a patent number #9,962,395 licensed (CleopatraRX), a patent number #10,369,158 licensed (CleopatraRX), and a patent number #10,758,496 issued; all patents are owned by the University of California, Los Angeles (UCLA), with R. Voskuhl’s role being an inventor on these UCLA patents. No other disclosures were reported.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

