Expanding SPTAN1 monoallelic variant associated disorders: From epileptic encephalopathy to pure spastic paraplegia and ataxia
- PMID: 36331550
- PMCID: PMC10620943
- DOI: 10.1016/j.gim.2022.09.013
Expanding SPTAN1 monoallelic variant associated disorders: From epileptic encephalopathy to pure spastic paraplegia and ataxia
Abstract
Purpose: Nonerythrocytic αII-spectrin (SPTAN1) variants have been previously associated with intellectual disability and epilepsy. We conducted this study to delineate the phenotypic spectrum of SPTAN1 variants.
Methods: We carried out SPTAN1 gene enrichment analysis in the rare disease component of the 100,000 Genomes Project and screened 100,000 Genomes Project, DECIPHER database, and GeneMatcher to identify individuals with SPTAN1 variants. Functional studies were performed on fibroblasts from 2 patients.
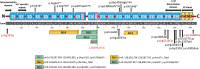
Results: Statistically significant enrichment of rare (minor allele frequency < 1 × 10-5) probably damaging SPTAN1 variants was identified in families with hereditary ataxia (HA) or hereditary spastic paraplegia (HSP) (12/1142 cases vs 52/23,847 controls, p = 2.8 × 10-5). We identified 31 individuals carrying SPTAN1 heterozygous variants or deletions. A total of 10 patients presented with pure or complex HSP/HA. The remaining 21 patients had developmental delay and seizures. Irregular αII-spectrin aggregation was noted in fibroblasts derived from 2 patients with p.(Arg19Trp) and p.(Glu2207del) variants.
Conclusion: We found that SPTAN1 is a genetic cause of neurodevelopmental disorder, which we classified into 3 distinct subgroups. The first comprises developmental epileptic encephalopathy. The second group exhibits milder phenotypes of developmental delay with or without seizures. The final group accounts for patients with pure or complex HSP/HA.
Keywords: Developmental delay; Developmental epileptic encephalopathy; Hereditary ataxia; Hereditary spastic paraplegia; SPTAN1.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no conflicts of interest.
Figures


References
-
- Miazek A., Zalas M., Skrzymowska J., et al. Age-dependent ataxia and neurodegeneration caused by an αII spectrin mutation with impaired regulation of its calpain sensitivity. Sci Rep. 2021;11(1):7312. doi: 10.1038/s41598-021-86470-1. Published correction appears in Sci Rep. 2021;11(1):18218. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

