The Role of Angiopoietins in Neovascular Diabetes-Related Retinal Diseases
- PMID: 36331711
- PMCID: PMC9663771
- DOI: 10.1007/s13300-022-01326-9
The Role of Angiopoietins in Neovascular Diabetes-Related Retinal Diseases
Abstract
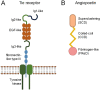
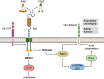
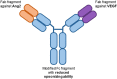
Diabetic retinopathy is a devastating and frequent complication of poorly controlled diabetes, whose pathogenesis is still only partially understood. Advances in basic research over the last two decades have led to the discovery of angiopoietins, proteins that strongly influence the growth and integrity of blood vessels in many vascular beds, with particular importance in the retina. Angiopoietin 1 (Ang1), produced mostly by pericytes and platelets, and angiopoietin 2 (Ang2), produced mainly by endothelial cells, bind to the same receptor (Tie2), but exert opposing effects on target cells. Ang1 maintains the stability of the mature vasculature, while Ang2 promotes vessel wall destabilization and disruption of the connections between endothelial cells and pericytes. Human retinal endothelial cells exposed to Ang2 show reduced membrane expression of the adhesion molecule VE-cadherin, and patients with proliferative diabetic retinopathy or diabetic macular edema have markedly increased vitreal concentrations of Ang2. Faricimab, a bi-specific antibody simultaneously directed against Ang2 and VEGF, has shown promising results in clinical trials among patients with diabetic retinopathy, and other agents targeting the angiopoietin system are currently in development.
Keywords: Angiopoietins; Diabetes complications; Faricimab; Macular edema; Retinopathy; Tie2.
© 2022. The Author(s).
Figures
References
-
- Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. - PubMed
-
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. - PubMed
-
- Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett. 2013;328:18–26. - PubMed
-
- Kim KT, Choi HH, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, Kim HZ, Lee GM, Koh GY. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem. 2005;280:20126–20131. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

