Dual-function artificial molecular motors performing rotation and photoluminescence
- PMID: 36332022
- PMCID: PMC9635830
- DOI: 10.1126/sciadv.add0410
Dual-function artificial molecular motors performing rotation and photoluminescence
Abstract
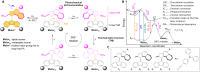
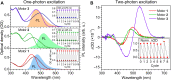
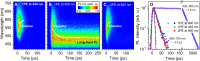
Molecular machines have caused one of the greatest paradigm shifts in chemistry, and by powering artificial mechanical molecular systems and enabling autonomous motion, they are expected to be at the heart of exciting new technologies. One of the biggest challenges that still needs to be addressed is designing the involved molecules to combine different orthogonally controllable functions. Here, we present a prototype of artificial molecular motors exhibiting the dual function of rotary motion and photoluminescence. Both properties are controlled by light of different wavelengths or by exploiting motors' outstanding two-photon absorption properties using low-intensity near-infrared light. This provides a noninvasive way to both locate and operate these motors in situ, essential for the application of molecular machines in complex (bio)environments.
Figures
References
-
- Sauvage J.-P., From chemical topology to molecular machines (Nobel lecture). Angew. Chem. Int. Ed. 56, 11080–11093 (2017). - PubMed
-
- C. J. Bruns, J. F. Stoddart, The Nature of the Mechanical Bond: From Molecules to Machines (John Wiley & Sons Inc., 2016).
-
- Browne W. R., Feringa B. L., Making molecular machines work. Nat. Nanotechnol. 1, 25–35 (2006). - PubMed
-
- J. P. Sauvage, P. Gaspard, From Non-Covalent Assemblies to Molecular Machines (Wiley-VCH Verlag GmbH & Co. KGaA, 2011).
-
- Pezzato C., Cheng C., Stoddart J. F., Astumian R. D., Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 46, 5491–5507 (2017). - PubMed
LinkOut - more resources
Full Text Sources

