Tumor-intrinsic SIRPA promotes sensitivity to checkpoint inhibition immunotherapy in melanoma
- PMID: 36332624
- PMCID: PMC9669221
- DOI: 10.1016/j.ccell.2022.10.012
Tumor-intrinsic SIRPA promotes sensitivity to checkpoint inhibition immunotherapy in melanoma
Abstract
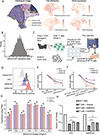
Checkpoint inhibition immunotherapy has revolutionized cancer treatment, but many patients show resistance. Here we perform integrative transcriptomic and proteomic analyses on emerging immuno-oncology targets across multiple clinical cohorts of melanoma under anti-PD-1 treatment, on both bulk and single-cell levels. We reveal a surprising role of tumor-intrinsic SIRPA in enhancing antitumor immunity, in contrast to its well-established role as a major inhibitory immune modulator in macrophages. The loss of SIRPA expression is a marker of melanoma dedifferentiation, a key phenotype linked to immunotherapy efficacy. Inhibition of SIRPA in melanoma cells abrogates tumor killing by activated CD8+ T cells in a co-culture system. Mice bearing SIRPA-deficient melanoma tumors show no response to anti-PD-L1 treatment, whereas melanoma-specific SIRPA overexpression significantly enhances immunotherapy response. Mechanistically, SIRPA is regulated by its pseudogene, SIRPAP1. Our results suggest a complicated role of SIRPA in the tumor ecosystem, highlighting cell-type-dependent antagonistic effects of the same target on immunotherapy.
Keywords: anti-PD-1 treatment; biomarker; immunotherapy; single-cell analysis; therapeutic target.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.L. is a shareholder and scientific adviser of Precision Scientific Ltd.
Figures






Comment in
-
Could SIRPA expression predict response to anti-PD-1 immunotherapy?Cancer Cell. 2022 Nov 14;40(11):1269-1270. doi: 10.1016/j.ccell.2022.10.003. Epub 2022 Nov 3. Cancer Cell. 2022. PMID: 36332626
References
-
- Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Dopp EA, Dijkstra CD, Simmons DL, and van den Berg TK (1998). Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol 161, 1853–1859. - PubMed
-
- Agaimy A, Specht K, Stoehr R, Lorey T, Markl B, Niedobitek G, Straub M, Hager T, Reis AC, Schilling B, et al. (2016). Metastatic Malignant Melanoma With Complete Loss of Differentiation Markers (Undifferentiated/Dedifferentiated Melanoma): Analysis of 14 Patients Emphasizing Phenotypic Plasticity and the Value of Molecular Testing as Surrogate Diagnostic Marker. Am J Surg Pathol 40, 181–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

