Development of the First Tritiated Tetrazine: Facilitating Tritiation of Proteins
- PMID: 36333105
- PMCID: PMC10100488
- DOI: 10.1002/cbic.202200539
Development of the First Tritiated Tetrazine: Facilitating Tritiation of Proteins
Abstract
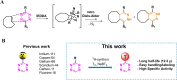
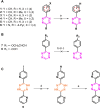
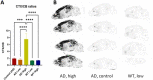
Tetrazine (Tz)-trans-cyclooctene (TCO) ligation is an ultra-fast and highly selective reaction and it is particularly suited to label biomolecules under physiological conditions. As such, a 3 H-Tz based synthon would have wide applications for in vitro/ex vivo assays. In this study, we developed a 3 H-labeled Tz and characterized its potential for application to pretargeted autoradiography. Several strategies were explored to synthesize such a Tz. However, classical approaches such as reductive halogenation failed. For this reason, we designed a Tz containing an aldehyde and explored the possibility of reducing this group with NaBT4 . This approach was successful and resulted in [3 H]-(4-(6-(pyridin-2-yl)-1,2,4,5-tetrazin-3-yl)phenyl)methan-t-ol with a radiochemical yield of 22 %, a radiochemical purity of 96 % and a molar activity of 0.437 GBq/μmol (11.8 Ci/mmol). The compound was successfully applied to pretargeted autoradiography. Thus, we report the synthesis of the first 3 H-labeled Tz and its successful application as a labeling building block.
Keywords: autoradiography; bioorthogonal chemistry; pre-targeting; tetrazine ligation; tritiation.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

