Effect of human mesenchymal stem cell secretome administration on morphine self-administration and relapse in two animal models of opioid dependence
- PMID: 36333316
- PMCID: PMC9636200
- DOI: 10.1038/s41398-022-02225-0
Effect of human mesenchymal stem cell secretome administration on morphine self-administration and relapse in two animal models of opioid dependence
Abstract
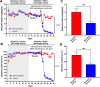
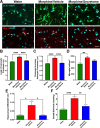
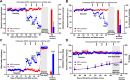
The present study investigates the possible therapeutic effects of human mesenchymal stem cell-derived secretome on morphine dependence and relapse. This was studied in a new model of chronic voluntary morphine intake in Wistar rats which shows classic signs of morphine intoxication and a severe naloxone-induced withdrawal syndrome. A single intranasal-systemic administration of MSCs secretome fully inhibited (>95%; p < 0.001) voluntary morphine intake and reduced the post-deprivation relapse intake by 50% (p < 0.02). Since several studies suggest a significant genetic contribution to the chronic use of many addictive drugs, the effect of MSCs secretome on morphine self-administration was further studied in rats bred as high alcohol consumers (UChB rats). Sub-chronic intraperitoneal administration of morphine before access to increasing concentrations of morphine solutions and water were available to the animals, led UChB rats to prefer ingesting morphine solutions over water, attaining levels of oral morphine intake in the range of those in the Wistar model. Intranasally administered MSCs secretome to UChB rats dose-dependently inhibited morphine self-administration by 72% (p < 0.001); while a single intranasal dose of MSC-secretome administered during a morphine deprivation period imposed on chronic morphine consumer UChB rats inhibited re-access morphine relapse intake by 80 to 85% (p < 0.0001). Both in the Wistar and the UChB rat models, MSCs-secretome administration reversed the morphine-induced increases in brain oxidative stress and neuroinflammation, considered as key engines perpetuating drug relapse. Overall, present preclinical studies suggest that products secreted by human mesenchymal stem cells may be of value in the treatment of opioid addiction.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. Opiate Overdose. https://www.whoint/news-room/fact-sheets/detail/opioid-overdose 2021.
-
- Harper S, Riddell CA, King NB. Declining Life Expectancy in the United States: Missing the Trees for the Forest. Annu Rev Public Health. 2021;42:381–403. - PubMed
-
- Ruhm CJ. Drivers of the fatal drug epidemic. J Health Econ. 2019;64:25–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

