Longitudinal analysis of the Five Sisters hot springs in Yellowstone National Park reveals a dynamic thermoalkaline environment
- PMID: 36333441
- PMCID: PMC9636164
- DOI: 10.1038/s41598-022-22047-w
Longitudinal analysis of the Five Sisters hot springs in Yellowstone National Park reveals a dynamic thermoalkaline environment
Abstract
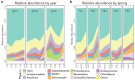
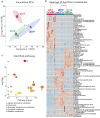
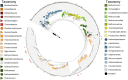
Research focused on microbial populations of thermoalkaline springs has been driven in a large part by the lure of discovering functional enzymes with industrial applications in high-pH and high temperature environments. While several studies have focused on understanding the fundamental ecology of these springs, the small molecule profiles of thermoalkaline springs have largely been overlooked. To better understand how geochemistry, small molecule composition, and microbial communities are connected, we conducted a three-year study of the Five Sisters (FS) springs that included high-resolution geochemical measurements, 16S rRNA sequencing of the bacterial and archaeal community, and mass spectrometry-based metabolite and extracellular small molecule characterization. Integration of the four datasets facilitated a comprehensive analysis of the interwoven thermoalkaline spring system. Over the course of the study, the microbial population responded to changing environmental conditions, with archaeal populations decreasing in both relative abundance and diversity compared to bacterial populations. Decreases in the relative abundance of Archaea were associated with environmental changes that included decreased availability of specific nitrogen- and sulfur-containing extracellular small molecules and fluctuations in metabolic pathways associated with nitrogen cycling. This multi-factorial analysis demonstrates that the microbial community composition is more closely correlated with pools of extracellular small molecules than with the geochemistry of the thermal springs. This is a novel finding and suggests that a previously overlooked component of thermal springs may have a significant impact on microbial community composition.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Mueller RC, Peach JT, Skorupa DJ, Copié V, Bothner B, Peyton BM. An emerging view of the diversity, ecology, and function of Archaea in alkaline hydrothermal environments. FEMS Microbiol. Ecol. 2020;97:fiaa246. - PubMed
-
- Patel AK, Singhania RR, Sim SJ, Pandey A. Thermostable cellulases: Current status and perspectives. Bioresour Technol. 2019;279:385–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

