Restoring After Central Nervous System Injuries: Neural Mechanisms and Translational Applications of Motor Recovery
- PMID: 36333482
- PMCID: PMC9723055
- DOI: 10.1007/s12264-022-00959-x
Restoring After Central Nervous System Injuries: Neural Mechanisms and Translational Applications of Motor Recovery
Abstract
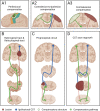
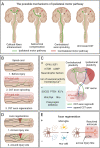
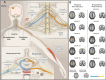
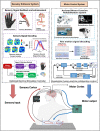
Central nervous system (CNS) injuries, including stroke, traumatic brain injury, and spinal cord injury, are leading causes of long-term disability. It is estimated that more than half of the survivors of severe unilateral injury are unable to use the denervated limb. Previous studies have focused on neuroprotective interventions in the affected hemisphere to limit brain lesions and neurorepair measures to promote recovery. However, the ability to increase plasticity in the injured brain is restricted and difficult to improve. Therefore, over several decades, researchers have been prompted to enhance the compensation by the unaffected hemisphere. Animal experiments have revealed that regrowth of ipsilateral descending fibers from the unaffected hemisphere to denervated motor neurons plays a significant role in the restoration of motor function. In addition, several clinical treatments have been designed to restore ipsilateral motor control, including brain stimulation, nerve transfer surgery, and brain-computer interface systems. Here, we comprehensively review the neural mechanisms as well as translational applications of ipsilateral motor control upon rehabilitation after CNS injuries.
Keywords: Axon regrowth; Brain–computer interface system; Ipsilateral motor control; Neuroplasticity; Spinal cord injury; Stroke; Traumatic brain injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

