Artificial Nerve Containing Stem Cells, Vascularity and Scaffold; Review of Our Studies
- PMID: 36333622
- PMCID: PMC9902426
- DOI: 10.1007/s12015-022-10467-0
Artificial Nerve Containing Stem Cells, Vascularity and Scaffold; Review of Our Studies
Abstract
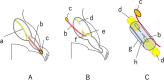
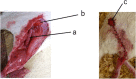
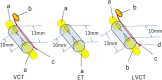
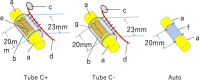
To promote nerve regeneration within a conduit (tubulation), we have performed studies using a tube model based on four important concepts for tissue engineering: vascularity, growth factors, cells, and scaffolds. A nerve conduit containing a blood vascular pedicle (vessel-containing tube) accelerated axon regeneration and increased the axon regeneration distance; however, it did not increase the number or diameter of the axons that regenerated within the tube. A vessel-containing tube with bone-marrow-derived mesenchymal stem cell (BMSC) transplantation led to the increase in the number and diameter of regenerated axons. Intratubularly transplanted decellularized allogenic nerve basal lamellae (DABLs) worked as a frame to maintain the fibrin matrix structure containing neurochemical factors and to anchor the transplanted stem cells within the tube. For the clinical application of nerve conduits, they should exhibit capillary permeability, biodegradability, and flexibility. Nerbridge® (Toyobo Co. Ltd., Osaka, Japan) is a commercially available artificial nerve conduit. The outer cylinder is a polyglycolic acid (PGA) fiber mesh and possesses capillary permeability. We used the outer cylinder of Nerbridge as a nerve conduit. A 20-mm sciatic nerve deficit was bridged by the PGA mesh tube containing DABLs and BMSCs, and the resulting nerve regeneration was compared with that obtained through a 20-mm autologous nerve graft. A neve-regeneration rate of about 70%-80% was obtained in 20-mm-long autologous nerve autografts using the new conduits.
Keywords: Artificial nerve; Axonal regeneration; Stem cells; Tissue engineering; Tubulation.
© 2022. The Author(s).
Conflict of interest statement
We have no competing interests with regard of this work.
Figures





References
-
- Kakinoki R, Nishijima N, Ueba Y, et al. Relationship between axonal regeneration and vascularity in tubulation—An experimental study in rats. Neuroscience Research. 1995;23(1):35–45. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

