Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway
- PMID: 36333786
- PMCID: PMC9636801
- DOI: 10.1186/s12974-022-02633-5
Fetuin-A alleviates neuroinflammation against traumatic brain injury-induced microglial necroptosis by regulating Nrf-2/HO-1 pathway
Abstract
Background: The microglia-mediated inflammatory response is a vital mechanism of secondary damage following traumatic brain injury (TBI), but the underlying mechanism of microglial activation is unclear.
Methods: Controlled cortical impact (CCI) was induced in adult male C57BL/6J mice, and glutamate was used to construct a classical in vitro injury model in the primary microglia. Microglial activation was determined by western blot and immunostaining. The inflammatory factors were measured by enzyme-linked immunosorbent assay. The oxidative stress marker and mitochondrial reactive oxygen species (ROS) were measured by immunoblotting and MitoSox Red staining. Transmission electron microscopy was used to observe the typical morphology of necroptotic cells.
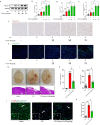
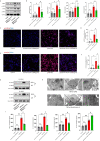
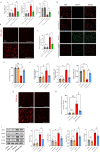
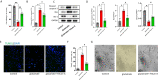
Results: Our quantitative proteomics identified 2499 proteins; 157 were significantly differentially expressed in brain tissue between the 6 h after CCI (CCI6h) group and sham group, and 109 were significantly differentially expressed between the CCI24h and sham groups. Moreover, compared with the sham group, the terms "acute-phase response", "inflammation", and "protein binding" were significantly enriched in CCI groups. Fetuin-A, a liver-secreted acute-phase glycoprotein, was involved in these biological processes. Using an experimental TBI model, we found that the Fetuin-A level peaked at 6 h and then decreased gradually. Importantly, we showed that administration of Fetuin-A reduced the cortical lesion volume and edema area and inhibited the inflammatory response, which was associated with suppressing microglial necroptosis, thus decreasing microglial activation. Furthermore, administration of Fetuin-A attenuated mitochondrial oxidative stress in glutamate-treated microglial cells, which is a critical mechanism of necroptosis suppression. In addition, we demonstrated that Fetuin-A treatment promoted translocation of nuclear factor erythroid 2-related factor 2 (Nrf-2) from the cytoplasm to the nucleus in vivo; however, the Nrf-2 inhibitor ML385 and si-heme oxygenase-1 (si-HO-1) disrupted the regulation of oxidative stress by Fetuin-A and induced increased ROS levels and necroptosis in glutamate-treated microglial cells. Fetuin-A also protected neurons from adverse factors in vivo and in vitro.
Conclusions: Our results demonstrated that Fetuin-A activated Nrf-2/HO-1, suppressed oxidative stress and necroptosis levels, and thereby attenuates the abnormal inflammatory response following TBI. The findings suggest a potential therapeutic strategy for TBI treatment.
Keywords: Fetuin-A; Microglia; Necroptosis; Neuroinflammation; Nrf-2/HO-1 pathway; Oxidative stress; Traumatic brain injury (TBI).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. - PubMed
-
- Shafi S, Barnes SA, Millar D, Sobrino J, Kudyakov R, Berryman C, Rayan N, Dubiel R, Coimbra R, Magnotti LJ, et al. Suboptimal compliance with evidence-based guidelines in patients with traumatic brain injuries. J Neurosurg. 2014;120:773–777. - PubMed
-
- Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A, Eynon CA, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375:1119–1130. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

