Cumulative incidence of SARS-CoV-2 infection in the general population of the Valencian Community (Spain) after the surge of the Omicron BA.1 variant
- PMID: 36333837
- PMCID: PMC9828341
- DOI: 10.1002/jmv.28284
Cumulative incidence of SARS-CoV-2 infection in the general population of the Valencian Community (Spain) after the surge of the Omicron BA.1 variant
Abstract
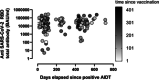
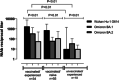
Studies investigating the cumulative incidence of and immune status against SARS-CoV-2 infection provide valuable information for shaping public health decision-making. A cross-sectional study on 935 participants, conducted in the Valencian Community (VC), measuring anti-SARS-CoV-2-receptor binding domain-RBD-total antibodies and anti-Nucleocapsid (N)-IgGs via electrochemiluminescence assays. Quantitation of neutralizing antibodies (NtAb) against ancestral and Omicron BA.1 and BA.2 variants and enumeration of SARS-CoV-2-S specific-IFNγ-producing CD4+ and CD8+ T cells was performed in 100 and 137 participants, respectively. The weighted cumulative incidence was 51.9% (95% confidence interval [CI]: 48.7-55.1) and was inversely related to age. Anti-RBD total antibodies were detected in 97% of participants; vaccinated and SARS-CoV-2-experienced (VAC-ex; n = 442) presented higher levels (p < 0.001) than vaccinated/naïve (VAC-n; n = 472) and nonvaccinated/experienced (UNVAC-ex; n = 63) subjects. Antibody levels correlated inversely with time elapsed since last vaccine dose in VAC-n (Rho, -0.52; p < 0.001) but not in VAC-ex (rho -0.02; p = 0.57). Heterologous booster shots resulted in increased anti-RBD antibody levels compared with homologous schedules in VAC-n, but not in VAC-ex. NtAbs against Omicron BA.1 were detected in 94%, 75%, and 50% of VAC-ex, VAC-n and UNVAC-ex groups, respectively. For Omicron BA.2, the figures were 97%, 84%, and 40%, respectively. SARS-CoV-2-S-reactive IFN-γ T cells were detected in 73%, 75%, and 64% of VAC-ex, VAC-n and UNVAC-ex, respectively. Median frequencies for both T-cell subsets were comparable across groups. In summary, by April 2022, around half of the VC population had been infected with SARS-CoV-2 and, due to extensive vaccination, displayed hybrid immunity.
Keywords: SARS-CoV-2; T cells; cumulative incidence of SARS-CoV-2 infection; neutralizing antibodies; seroprevalence.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Spanish Center for the Coordination of Health Alerts and Emergencies . Update no. 605. Coronavirus Disease (COVID‐19). Accessed June 9, 2022. https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActu...
-
- Ministry of Health. GIV Covid 19 Comprehensive management of Covid vaccination. Activity report 03.06.2022. Spanish Ministry of Health, 2022. https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActu...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

