The Effect of Zoledronic Acid on Bone Microarchitecture and Strength after Denosumab and Teriparatide Administration: DATA-HD Study Extension
- PMID: 36333954
- PMCID: PMC10098948
- DOI: 10.1002/jbmr.4737
The Effect of Zoledronic Acid on Bone Microarchitecture and Strength after Denosumab and Teriparatide Administration: DATA-HD Study Extension
Abstract
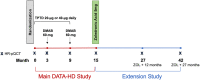
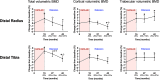
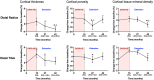
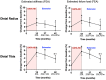
The combination of denosumab and teriparatide is an effective treatment strategy in postmenopausal osteoporosis, though skeletal gains are promptly lost when these agents are discontinued. In the DATA-HD study, we reported that a single dose of zoledronic acid (ZOL) maintains the increases in areal spine and hip bone mineral density (BMD) achieved with this combination for at least 12 months. The capacity of ZOL to maintain corresponding improvements in peripheral volumetric BMD and microarchitecture, however, has not been reported. In the 15-month DATA-HD study, 76 postmenopausal osteoporotic women were randomized to receive 9 months of teriparatide (20-μg or 40-μg daily) overlapped with denosumab (60 mg at months 3 and 9). In the Extension study, 53 participants received a single dose of ZOL (5 mg intravenously) 24-35 weeks after the last denosumab dose. We measured volumetric BMD and microarchitecture at the distal radius and tibia using high-resolution peripheral quantitative computed tomography at months 27 and 42. Despite ZOL administration, total and cortical BMD gradually decreased over 27 months resulting in values similar to baseline at the radius but still significantly above baseline at the tibia. At both sites, cortical porosity decreased to values below pretreatment baseline at month 27 but then increased from month 27 to 42. There were no significant changes in trabecular parameters throughout the 27-month post-ZOL observation period. Stiffness and failure load, at both sites, decreased progressively from month 15 42 though remained above baseline at the tibia. These findings suggest that in contrast to the largely maintained gains in dual-energy X-ray absorptiometry (DXA)-derived spine and hip BMD, a single dose of ZOL was not as effective in maintaining the gains in volumetric peripheral bone density and microarchitecture produced by 15 months of overlapping treatment with denosumab and teriparatide. Alternative therapeutic approaches that can fully maintain improvements in peripheral bone parameters require further study. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANABOLICS THERAPEUTICS; ANALYSIS/QUANTITATION OF BONE; ANTIRESORPTIVES THERAPEUTICS; CLINICAL TRIALS; DISEASES AND DISORDERS OF/RELATED TO BONE; OSTEOPOROSIS.
© 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

