Changes in breast cancer treatment during the COVID-19 pandemic: a Dutch population-based study
- PMID: 36334188
- PMCID: PMC9638417
- DOI: 10.1007/s10549-022-06732-y
Changes in breast cancer treatment during the COVID-19 pandemic: a Dutch population-based study
Abstract
Purpose: We aimed to compare (1) treatments and time intervals between treatments of breast cancer patients diagnosed during and before the COVID-19 pandemic, and (2) the number of treatments started during and before the pandemic.
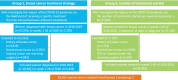
Methods: Women were selected from the Netherlands Cancer Registry. For aim one, odds ratios (OR) and 95% confidence intervals (95%CI) were calculated to compare the treatment of women diagnosed within four periods of 2020: pre-COVID (weeks 1-8), transition (weeks 9-12), lockdown (weeks 13-17), and care restart (weeks 18-26), with data from 2018/2019 as reference. Wilcoxon rank-sums test was used to compare treatment intervals, using a two-sided p-value < 0.05. For aim two, number of treatments started per week in 2020 was compared with 2018/2019.
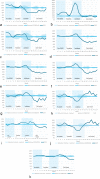
Results: We selected 34,097 women for aim one. Compared to 2018/2019, neo-adjuvant chemotherapy was less likely for stage I (OR 0.24, 95%CI 0.11-0.53), stage II (OR 0.63, 95%CI 0.47-0.86), and hormone receptor+/HER2- tumors (OR 0.55, 95%CI 0.41-0.75) diagnosed during transition. Time between diagnosis and first treatment decreased for patients diagnosed during lockdown with a stage I (p < 0.01), II (p < 0.01) or III tumor (p = 0.01). We selected 30,002 women for aim two. The number of neo-adjuvant endocrine therapies and surgeries starting in week 14, 2020, increased by 339% and 18%, respectively. The number of adjuvant chemotherapies decreased by 42% in week 15 and increased by 44% in week 22.
Conclusion: The pandemic and subsequently altered treatment recommendations affected multiple aspects of the breast cancer treatment strategy and the number of treatments started per week.
Keywords: Breast cancer; Breast cancer care; COVID-19; Treatment.
© 2022. The Author(s).
Conflict of interest statement
Financial interests: HMV received funding by the Dutch Cancer Foundation, European Commission, ZonMw. MLS received grants from Servier Pharma and Nutricia. JW received funding from the Cancer Research UK KWF Dutch Cancer Society ZonMW and the Antoni van Leeuwenhoek Foundation. Non-financial interests: JW is a member of the Scientific Advisory Board Dutch Expert Centre for Screening. All other authors have no relevant financial or non-financial interests to disclose.
Figures


References
-
- NOS (2020) Ziekenhuizen hervatten reguliere zorg 'stap voor stap'. https://nos.nl/artikel/2334679-ziekenhuizen-hervatten-reguliere-zorg-sta.... Accessed 16 July 2021
-
- Association of breast surgery (2020) Statement from the association of breast surgery, 15th March 2020. https://associationofbreastsurgery.org.uk/media/252009/abs-statement-150.... Accessed 28 Sept 2021
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

