NCX2 Regulates Intracellular Calcium Homeostasis and Translocation of HIF-1α into the Nucleus to Inhibit Glioma Invasion
- PMID: 36334237
- PMCID: PMC10199856
- DOI: 10.1007/s10528-022-10274-9
NCX2 Regulates Intracellular Calcium Homeostasis and Translocation of HIF-1α into the Nucleus to Inhibit Glioma Invasion
Abstract
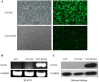
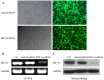
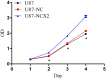
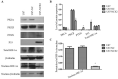
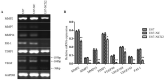
Glioma is the most common tumor of the central nervous system, and its poor prognosis can be linked to hypoxia and gene inactivation. Na+/Ca2+ exchanger 2 (NCX2) is expressed only in the normal brain and not in other tissues or glioma. We constructed a hypoxic microenvironment to more accurately understand the effect of NCX2 in glioma. Our previous experiments confirmed that NCX2 inhibited the growth of U87 cells in nude mice, indicating that NCX2 is a potential tumor suppressor gene. Malignant tumor cells are often exposed to an anoxic environment. To more accurately understand the effect of NCX2 in glioma, we constructed a hypoxic microenvironment. To detect the localization of NCX2 in transfected U87 cells, immunofluorescence was used. We tested the function of NCX2 in glioma, i.e., how it contributes to the cytosolic Ca2+ homeostasis by X-Rhod-1. We tested the cell proliferation of NCX2 in glioma in hypoxic using Cell counting kit-8 (CCK8). Cell migration and invasion were evaluated in 24-well transwell matrigel-coated or non-matrigel-coated in hypoxia. NCX2 promoted the proliferation of U87 cells in the hypoxic microenvironment. It inhibited the invasion and migration abilities of U87 cells. We demonstrated that NCX2 was located on the cell membrane and that it reduced intracellular Ca2+ levels and reactivated P53 and PTEN. We further demonstrated that NCX2 impaired cell invasion through the HIF-1α pathway in glioma. The results indicated that NCX2 plays a key role in glioma formation and tumor invasion functionality.
Keywords: Glioma; HIF-1α; Hypoxic; Invasion; NCX2.
© 2022. The Author(s).
Conflict of interest statement
The authors have not disclosed any competing interests.
Figures



References
-
- Andrikopoulos P, Baba A, Matsuda T, Djamgoz MBA, Yaqoob MM, Eccles SA. Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem. 2011;286(44):37919–37931. doi: 10.1074/jbc.M111.251777. - DOI - PMC - PubMed
-
- Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

