Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus gallus): Multi-omics reveals critical role of gut-liver axis
- PMID: 36334886
- PMCID: PMC10555772
- DOI: 10.1016/j.jare.2022.10.015
Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus gallus): Multi-omics reveals critical role of gut-liver axis
Abstract
Introduction: Microplastic pollution seriously threatens the health and safety of humans and wildlife. Avian is one of the main species endangered by microplastics. However, the damage mechanism of microplastics to the digestive system of avian is not clear.
Objectives: The gut-liver axis is a bidirectional channel that regulates the exchange of information between the gut and the liver and is also a key target for tissue damage caused by pollutants. This study aimed to elucidate the digestive toxicity of microplastics in avian and the key role of the gut-liver axis in it.
Methods: We constructed an exposure model for microplastics in environmental concentrations and toxicological concentrations in chickens and reveal the digestive toxicity of polystyrene microplastics (PS-MPs) in avian by 16S rRNA, transcriptomics and metabolomics.
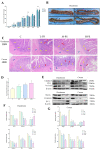
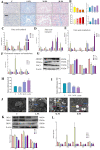
Results: PS-MPs changed the death mode from apoptosis to necrosis and pyroptosis by upregulating Caspase 8, disrupting the intestinal vascular barrier, disturbing the intestinal flora and promoting the accumulation of lipopolysaccharide. Harmful flora and metabolites were translocated to the liver through the liver-gut axis, eliciting hepatic immune responses and promoting hepatic lipid metabolism disorders and apoptosis. Liver injury involves multiple molecular effects of mitochondrial dynamics disturbance, oxidative stress, endoplasmic reticulum stress, and cell cycle disturbance. Furthermore, metabolomics suggested that caffeine and melanin metabolites may be potential natural resistance substances for microplastics.
Conclusion: Taken together, our data demonstrate the digestive damage of PS-MPs in avian, revealing a critical role of the liver-gut axis in it. This will provide a reference for protecting the safety of avian populations.
Keywords: Avian; Gut vascular barrier; Gut–liver axis; Microplastic; Multi-omics analysis.
Copyright © 2021. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Lindeque P.K., Cole M., Coppock R.L., Lewis C.N., Miller R.Z., Watts A.J.R., et al. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ Pollut. 2020;265:12. doi: 10.1016/j.envpol.2020.114721. - DOI - PubMed
-
- Horton A.A., Walton A., Spurgeon D.J., Lahive E., Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

