Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy
- PMID: 36335091
- PMCID: PMC9637113
- DOI: 10.1038/s41419-022-05364-w
Cadmium-induced apoptosis of Leydig cells is mediated by excessive mitochondrial fission and inhibition of mitophagy
Abstract
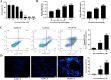
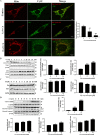
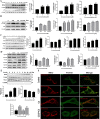
Cadmium is one of the environmental and occupational pollutants and its potential adverse effects on human health have given rise to substantial concern. Cadmium causes damage to the male reproductive system via induction of germ-cell apoptosis; however, the underlying mechanism of cadmium-induced reproductive toxicity in Leydig cells remains unclear. In this study, twenty mice were divided randomly into four groups and exposed to CdCl2 at concentrations of 0, 0.5, 1.0 and 2.0 mg/kg/day for four consecutive weeks. Testicular injury, abnormal spermatogenesis and apoptosis of Leydig cells were observed in mice. In order to investigate the mechanism of cadmium-induced apoptosis of Leydig cells, a model of mouse Leydig cell line (i.e. TM3 cells) was subjected to treatment with various concentrations of CdCl2. It was found that mitochondrial function was disrupted by cadmium, which also caused a significant elevation in levels of mitochondrial superoxide and cellular ROS. Furthermore, while cadmium increased the expression of mitochondrial fission proteins (DRP1 and FIS1), it reduced the expression of mitochondrial fusion proteins (OPA1 and MFN1). This led to excessive mitochondrial fission, the release of cytochrome c and apoptosis. Conversely, cadmium-induced accumulation of mitochondrial superoxide was decreased by the inhibition of mitochondrial fission through the use of Mdivi-1 (an inhibitor of DRP1). Mdivi-1 also partially prevented the release of cytochrome c from mitochondria to cytosol and attenuated cell apoptosis. Finally, given the accumulation of LC3II and SQSTM1/p62 and the obstruction of Parkin recruitment into damaged mitochondria in TM3 cells, the autophagosome-lysosome fusion was probably inhibited by cadmium. Overall, these findings suggest that cadmium induces apoptosis of mouse Leydig cells via the induction of excessive mitochondrial fission and inhibition of mitophagy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology. 2016;4:648–61. - PubMed
-
- Hong H, Xu Y, Xu J, Zhang J, Xi Y, Pi H, et al. Cadmium exposure impairs pancreatic beta-cell function and exaggerates diabetes by disrupting lipid metabolism. Environ Int. 2021;149:106406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

