Ferroptosis: a double-edged sword mediating immune tolerance of cancer
- PMID: 36335094
- PMCID: PMC9637147
- DOI: 10.1038/s41419-022-05384-6
Ferroptosis: a double-edged sword mediating immune tolerance of cancer
Abstract
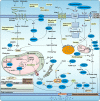
The term ferroptosis was put forward in 2012 and has been researched exponentially over the past few years. Ferroptosis is an unconventional pattern of iron-dependent programmed cell death, which belongs to a type of necrosis and is distinguished from apoptosis and autophagy. Actuated by iron-dependent phospholipid peroxidation, ferroptosis is modulated by various cellular metabolic and signaling pathways, including amino acid, lipid, iron, and mitochondrial metabolism. Notably, ferroptosis is associated with numerous diseases and plays a double-edged sword role. Particularly, metastasis-prone or highly-mutated tumor cells are sensitive to ferroptosis. Hence, inducing or prohibiting ferroptosis in tumor cells has vastly promising potential in treating drug-resistant cancers. Immunotolerant cancer cells are not sensitive to the traditional cell death pathway such as apoptosis and necroptosis, while ferroptosis plays a crucial role in mediating tumor and immune cells to antagonize immune tolerance, which has broad prospects in the clinical setting. Herein, we summarized the mechanisms and delineated the regulatory network of ferroptosis, emphasized its dual role in mediating immune tolerance, proposed its significant clinical benefits in the tumor immune microenvironment, and ultimately presented some provocative doubts. This review aims to provide practical guidelines and research directions for the clinical practice of ferroptosis in treating immune-resistant tumors.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

