Molecular profile and its clinical impact of IDH1 mutated versus IDH1 wild type intrahepatic cholangiocarcinoma
- PMID: 36335135
- PMCID: PMC9637171
- DOI: 10.1038/s41598-022-22543-z
Molecular profile and its clinical impact of IDH1 mutated versus IDH1 wild type intrahepatic cholangiocarcinoma
Abstract
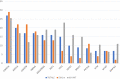
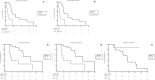
IDH1-mutated cholangiocarcinomas (CCAs) are an interesting group of neoplasia with particular behavior and therapeutic implications. The aim of the present work is to highlight the differences characterizing IDH1m and IDH1wt CCAs in terms of genomic landscape. 284 patients with iCCA treated for resectable, locally advanced or metastatic disease were selected and studied with the FOUNDATION Cdx technology. A comparative genomic analysis and survival analyses for the most relevant altered genes were performed between IDH1m and IDH1wt patients. Overall, 125 patients were IDH1m and 122 IDH1wt. IDH1m patients showed higher mutation rates compared to IDH1wt in CDKN2B and lower mutation rates in several genes including TP53, FGFR2, BRCA2, ATM, MAP3K1, NOTCH2, ZNF703, CCND1, NBN, NF1, MAP3KI3, and RAD21. At the survival analysis, IDH1m and IDH1wt patients showed no statistically differences in terms of survival outcomes, but a trend in favor of IDH1wt patients was observed. Differences in prognostic values of the most common altered genes were reported. In surgical setting, in IDH1m group the presence of CDKN2A and CDKN2B mutations negatively impact DFS, whereas the presence of CDKN2A, CDKN2B, and PBRM1 mutations negatively impact OS. In advanced setting, in the IDH1m group, the presence of KRAS/NRAS and TP53 mutations negatively impact PFS, whereas the presence of TP53 and PIK3CA mutations negatively impact OS; in the IDH1wt group, only the presence of MTAP mutation negatively impact PFS, whereas the presence of TP53 mutation negatively impact OS. We highlighted several molecular differences with distinct prognostic implications between IDH1m and IDH1wt patients.
© 2022. The Author(s).
Conflict of interest statement
MN: Travel expenses from Celgene, speaker honorarium from Accademia della Medicina. Consultant honoraria from EMD Serono, Basilea Pharmaceutica, Incyte and MSD Italia. FdB: Honoraria from Roche, Pfizer, BMS, Merck, MSD, SERVIER, Sanofi, Amgen Astellas BioPharma, Incyte. Consulting or Advisory Role for Roche, Incyte, EMD Serono, BMS, Nerviano Medical Sciences, Sanofi, Novartis Italy, Menarini, research funding (institution): Novartis, Roche, Merck Serono, Pfizer, Servier, Philogen, Loxo, Tesaro, Nerviano Medical Sciences, Kymab. Research funding: BMS/Medarex, Merck KGaA, Ignyta, MedImmune, Exelis, Bayer health, Daiichi Sangyo Europe GmbH, Incyte, Basilea Pharmaceutical, jassen Oncology. TM: (SOBI) Swedish Orpahn Biovitrum AB, Ability Pharmaceuticals SL, Aptitude Health, AstraZeneca, Basilea Pharma, Baxter, BioLineRX Ltd, Celgene, Eisai, Ellipses, Genzyme, Got It Consulting SL, Hirslanden/GITZ, Imedex, Incyte, Ipsen Bioscience , Inc, Janssen, Lilly. Marketing Farmacéutico & Investigación Clínica, S.L, MDS, Medscape, Novocure, Paraxel, PPD Development, Polaris, QED Therapeutics, Roche Farma, Sanofi-Aventis, Servier, Scilink Comunicación Científica SC, Surface Oncology, and Zymeworks. All other authors declare no competing interests.
Figures


Similar articles
-
Clinical Outcomes After Progression on First-Line Therapies in IDH1 Mutated Versus Wild-Type Intrahepatic Cholangiocarcinoma Patients.Target Oncol. 2023 Jan;18(1):139-145. doi: 10.1007/s11523-022-00933-7. Epub 2023 Jan 23. Target Oncol. 2023. PMID: 36689074
-
Next-generation sequencing analysis of cholangiocarcinoma identifies distinct IDH1-mutated clusters.Eur J Cancer. 2022 Nov;175:299-310. doi: 10.1016/j.ejca.2022.08.026. Epub 2022 Sep 28. Eur J Cancer. 2022. PMID: 36182816
-
Platinum sensitivity in patients with IDH1/2 mutated vs wild-type intrahepatic cholangiocarcinoma: A propensity score-based study.Int J Cancer. 2022 Oct 15;151(8):1310-1320. doi: 10.1002/ijc.34182. Epub 2022 Jul 9. Int J Cancer. 2022. PMID: 35723131
-
Mutations of isocitrate dehydrogenase 1 and 2 in intrahepatic cholangiocarcinoma.Curr Opin Gastroenterol. 2014 May;30(3):295-302. doi: 10.1097/MOG.0000000000000050. Curr Opin Gastroenterol. 2014. PMID: 24569570 Review.
-
Ivosidenib in IDH1-mutated cholangiocarcinoma: Clinical evaluation and future directions.Pharmacol Ther. 2022 Sep;237:108170. doi: 10.1016/j.pharmthera.2022.108170. Epub 2022 Mar 13. Pharmacol Ther. 2022. PMID: 35296436 Review.
Cited by
-
Isocitrate dehydrogenase 1 gene mutations: a case review unveiling its biological impact on disease progression, prognosis and treatment in Chilean patients.BJR Case Rep. 2025 Mar 24;11(2):uaaf019. doi: 10.1093/bjrcr/uaaf019. eCollection 2025 Mar. BJR Case Rep. 2025. PMID: 40182999 Free PMC article. Review.
-
Long-Term Survivor of Intrahepatic Cholangiocarcinoma for over 18 Years: Case Study with Longitudinal Histo-molecular and Tumor Immune Microenvironment Characterization and Systematic Review of the Literature.J Gastrointest Cancer. 2024 Dec;55(4):1634-1646. doi: 10.1007/s12029-024-01113-8. Epub 2024 Sep 16. J Gastrointest Cancer. 2024. PMID: 39283582 Free PMC article.
-
Updated survival outcomes with ivosidenib in patients with previously treated IDH1-mutated intrahepatic-cholangiocarcinoma: an Italian real-world experience.Ther Adv Med Oncol. 2023 Jul 12;15:17588359231171574. doi: 10.1177/17588359231171574. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37457302 Free PMC article.
-
Unveil Intrahepatic Cholangiocarcinoma Heterogeneity through the Lens of Omics and Multi-Omics Approaches.Cancers (Basel). 2024 Aug 20;16(16):2889. doi: 10.3390/cancers16162889. Cancers (Basel). 2024. PMID: 39199659 Free PMC article. Review.
-
Durvalumab Plus Gemcitabine and Cisplatin Versus Gemcitabine and Cisplatin in Biliary Tract Cancer: a Real-World Retrospective, Multicenter Study.Target Oncol. 2024 May;19(3):359-370. doi: 10.1007/s11523-024-01060-1. Epub 2024 May 1. Target Oncol. 2024. PMID: 38691295
References
-
- Howlader, N., Noone, A.M., Krapcho, M., et al. (Eds.) SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, Based on November 2015 SEER Data Submission, Posted to the SEER Web Site; April 2016. http://seer.cancer.gov/csr/1975_2013/. Accessed 10 Dec 2016 (2016).
-
- Rimini M, Puzzoni M, Pedica F, Silvestris N, Fornaro L, Aprile G, Loi E, Brunetti O, Vivaldi C, Simionato F, Zavattari P, Scartozzi M, Burgio V, Ratti F, Aldrighetti L, Cascinu S, Casadei-Gardini A. Cholangiocarcinoma: New perspectives for new horizons. Exp. Rev. Gastroenterol. Hepatol. 2021;15(12):1367–1383. doi: 10.1080/17474124.2021.1991313. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

