The P. aeruginosa effector Tse5 forms membrane pores disrupting the membrane potential of intoxicated bacteria
- PMID: 36335275
- PMCID: PMC9637101
- DOI: 10.1038/s42003-022-04140-y
The P. aeruginosa effector Tse5 forms membrane pores disrupting the membrane potential of intoxicated bacteria
Abstract
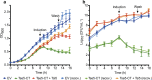
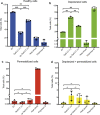
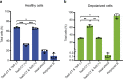
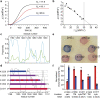
The type VI secretion system (T6SS) of Pseudomonas aeruginosa injects effector proteins into neighbouring competitors and host cells, providing a fitness advantage that allows this opportunistic nosocomial pathogen to persist and prevail during the onset of infections. However, despite the high clinical relevance of P. aeruginosa, the identity and mode of action of most P. aeruginosa T6SS-dependent effectors remain to be discovered. Here, we report the molecular mechanism of Tse5-CT, the toxic auto-proteolytic product of the P. aeruginosa T6SS exported effector Tse5. Our results demonstrate that Tse5-CT is a pore-forming toxin that can transport ions across the membrane, causing membrane depolarisation and bacterial death. The membrane potential regulates a wide range of essential cellular functions; therefore, membrane depolarisation is an efficient strategy to compete with other microorganisms in polymicrobial environments.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Acquisition of T6SS Effector TseL Contributes to the Emerging of Novel Epidemic Strains of Pseudomonas aeruginosa.Microbiol Spectr. 2023 Feb 14;11(1):e0330822. doi: 10.1128/spectrum.03308-22. Epub 2022 Dec 22. Microbiol Spectr. 2023. PMID: 36546869 Free PMC article.
-
Electrophysiological dissection of the ion channel activity of the Pseudomonas aeruginosa ionophore protein toxin Tse5.Chem Phys Lipids. 2025 Mar;267:105472. doi: 10.1016/j.chemphyslip.2025.105472. Epub 2025 Jan 6. Chem Phys Lipids. 2025. PMID: 39778700
-
A family of Type VI secretion system effector proteins that form ion-selective pores.Nat Commun. 2019 Dec 2;10(1):5484. doi: 10.1038/s41467-019-13439-0. Nat Commun. 2019. PMID: 31792213 Free PMC article.
-
Specialized killing across the domains of life by the type VI secretion systems of Pseudomonas aeruginosa.Biochem J. 2025 Jan 8;482(1):1-15. doi: 10.1042/BCJ20230240. Biochem J. 2025. PMID: 39774785 Free PMC article. Review.
-
The T6SSs of Pseudomonas aeruginosa Strain PAO1 and Their Effectors: Beyond Bacterial-Cell Targeting.Front Cell Infect Microbiol. 2016 Jun 9;6:61. doi: 10.3389/fcimb.2016.00061. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27376031 Free PMC article. Review.
Cited by
-
Two cysteines control Tse1 secretion by H1-T6SS in Pseudomonas aeruginosa.Protein Sci. 2025 Aug;34(8):e70226. doi: 10.1002/pro.70226. Protein Sci. 2025. PMID: 40719405 Free PMC article.
-
Acquisition of T6SS Effector TseL Contributes to the Emerging of Novel Epidemic Strains of Pseudomonas aeruginosa.Microbiol Spectr. 2023 Feb 14;11(1):e0330822. doi: 10.1128/spectrum.03308-22. Epub 2022 Dec 22. Microbiol Spectr. 2023. PMID: 36546869 Free PMC article.
-
Distribution of the four type VI secretion systems in Pseudomonas aeruginosa and classification of their core and accessory effectors.Nat Commun. 2025 Jan 21;16(1):888. doi: 10.1038/s41467-024-54649-5. Nat Commun. 2025. PMID: 39837841 Free PMC article.
-
Defining Membrane Protein Topology Using pho-lac Reporter Fusions.Methods Mol Biol. 2024;2715:181-195. doi: 10.1007/978-1-0716-3445-5_11. Methods Mol Biol. 2024. PMID: 37930528
-
Not just passengers: effectors contribute to the assembly of the type VI secretion system as structural building blocks.J Bacteriol. 2025 Mar 20;207(3):e0045524. doi: 10.1128/jb.00455-24. Epub 2025 Feb 4. J Bacteriol. 2025. PMID: 39902958 Free PMC article. Review.
References
-
- Bingle LE, Bailey CM, Pallen MJ. Type VI secretion: a beginner’s guide. Curr. Opin. Microbiol. 2008;11:3–8. - PubMed
-
- Pukatzki S, McAuley SB, Miyata ST. The type VI secretion system: translocation of effectors and effector-domains. Curr. Opin. Microbiol. 2009;12:11–17. - PubMed
-
- Cianfanelli FR, Monlezun L, Coulthurst SJ. Aim, load, fire: the Type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 2016;24:51–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

