Quality assessment of Clinical Practice Guidelines (CPG) for the diagnosis and treatment of inflammatory bowel disease using the AGREE II instrument: a systematic review
- PMID: 36335292
- PMCID: PMC9637309
- DOI: 10.1186/s12876-022-02539-9
Quality assessment of Clinical Practice Guidelines (CPG) for the diagnosis and treatment of inflammatory bowel disease using the AGREE II instrument: a systematic review
Abstract
Background: The incidence and diagnosis of inflammatory bowel disease (IBD) has increased considerably in recent years. Many clinical practice guidelines (CPG) have been developed for the management of this disease across different clinical contexts, however, little evidence exists on their methodological quality. Therefore, we aimed to systematically evaluate the quality of CPGs for the diagnosis and treatment of IBD using the Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument.
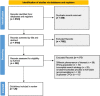
Methods: We identified CPGs by searching databases (MEDLINE - PubMed, EMBASE, CINAHL, LILACS) and other sources of gray literature on January 2022. We included guidelines with specific recommendations for the diagnosis and treatment of IBD and evaluated them with the AGREE II instrument to assess their methodological quality. Six independent reviewers assessed the quality of the guidelines and resolved conflicts by consensus. We assessed the degree of agreement using the intraclass correlation coefficient (ICC) and change in quality over time was appraised in two periods: from 2012 to 2017 and from 2018 to 2022.
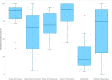
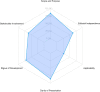
Results: We analyzed and evaluated 26 CPGs that met the inclusion criteria. The overall agreement among reviewers was moderate (ICC: 0.74; 95% CI 0.36 - 0.89). The mean scores of the AGREE II domains were: "Scope and purpose" 84.51%, "Stakeholder involvement" 60.90%, "Rigor of development" 69.95%, "Clarity of presentation" 85.58%, "Applicability" 26.60%, and "Editorial independence" 62.02%. No changes in quality were found over time.
Conclusions: The quality of the CPGs evaluated was generally good, with a large majority of the assessed guidelines being "recommended" and "recommended with modifications"; despite this, there is still room for improvement, especially in terms of stakeholder involvement and applicability. Efforts to develop high quality CPGs for IBD need to be further optimized.
Keywords: Clinical practice guidelines; Crohn disease; Inflammatory bowel disease; Systematic review; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests
Figures
Similar articles
-
Complementary and alternative medicine mention and recommendations in inflammatory bowel disease guidelines: systematic review and assessment using AGREE II.BMC Complement Med Ther. 2023 Jul 11;23(1):230. doi: 10.1186/s12906-023-04062-0. BMC Complement Med Ther. 2023. PMID: 37434218 Free PMC article.
-
A critical appraisal of urolithiasis clinical practice guidelines using the AGREE II instrument.Transl Androl Urol. 2023 Jun 30;12(6):977-988. doi: 10.21037/tau-22-846. Epub 2023 Jun 13. Transl Androl Urol. 2023. PMID: 37426603 Free PMC article.
-
Quality appraisal of clinical practice guidelines addressing massage interventions using the AGREE II instrument.Syst Rev. 2024 Mar 8;13(1):83. doi: 10.1186/s13643-024-02503-6. Syst Rev. 2024. PMID: 38459534 Free PMC article.
-
Quality assessment of clinical guidelines on probiotics therapy in children with IBD using the AGREE II instrument.J Clin Pharm Ther. 2021 Aug;46(4):1155-1165. doi: 10.1111/jcpt.13422. Epub 2021 Mar 25. J Clin Pharm Ther. 2021. PMID: 33768598
-
Treatment Guidelines for Atopic Dermatitis Since the Approval of Dupilumab: A Systematic Review and Quality Appraisal Using AGREE-II.Front Med (Lausanne). 2022 Mar 9;9:821871. doi: 10.3389/fmed.2022.821871. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35355606 Free PMC article.
Cited by
-
Clinical practice guidelines for the nutrition of colorectal cancer patients: a systematic review.Support Care Cancer. 2024 Feb 24;32(3):187. doi: 10.1007/s00520-024-08394-6. Support Care Cancer. 2024. PMID: 38396102
-
Hereditary colorectal cancer syndromes and inflammatory bowel disease: results from a registry-based study.Int J Colorectal Dis. 2025 Jan 25;40(1):24. doi: 10.1007/s00384-025-04808-x. Int J Colorectal Dis. 2025. PMID: 39863767 Free PMC article.
-
Complementary and alternative medicine mention and recommendations in inflammatory bowel disease guidelines: systematic review and assessment using AGREE II.BMC Complement Med Ther. 2023 Jul 11;23(1):230. doi: 10.1186/s12906-023-04062-0. BMC Complement Med Ther. 2023. PMID: 37434218 Free PMC article.
-
Appraisal of current surgical guidelines for inflammatory bowel disease using the AGREE-S instrument: A scoping review.Colorectal Dis. 2024 Dec 10;27(1):e17258. doi: 10.1111/codi.17258. Online ahead of print. Colorectal Dis. 2024. PMID: 39658521 Free PMC article.
-
GP preferences for, access to, and use of evidence in clinical practice: a mixed-methods study.BJGP Open. 2023 Dec 19;7(4):BJGPO.2023.0107. doi: 10.3399/BJGPO.2023.0107. Print 2023 Dec. BJGP Open. 2023. PMID: 37442591 Free PMC article.
References
-
- Yamamoto-Furushoa K, Bosques-Padilla B, de-Paula J, Galiano MT, P. Ibañez P, Juliao F, et al. Diagnosis and treatment of inflammatory bowel disease: First Latin American Consensus of the Pan American Crohn's sand Colitis Organisation. Rev Mex Med. 2017;82(1):46–84. doi: 10.1016/j.rgmx.2016.07.003. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

